1) Which of the following correctly lists the atoms in order of
... 1) An unknown acid reacts with NaOH according to the equation, H2X(s) + 2 NaOH(aq) → 2 H2O(l) + Na2X(aq). What is the molar mass of the acid if 22.4 mL of 0.0848 M NaOH is needed to neutralize 0.1846 g of the acid? 2) Calculate the maximum heat evolved when 4.61 grams C2H5OH (46.0684 g/mol) and 9.24 ...
... 1) An unknown acid reacts with NaOH according to the equation, H2X(s) + 2 NaOH(aq) → 2 H2O(l) + Na2X(aq). What is the molar mass of the acid if 22.4 mL of 0.0848 M NaOH is needed to neutralize 0.1846 g of the acid? 2) Calculate the maximum heat evolved when 4.61 grams C2H5OH (46.0684 g/mol) and 9.24 ...
Chapter 3 Discovering the atom and subatomic particles (History of
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
Biol 1020 Ch. 2 Chemistry
... Oxidation-Reduction Reactions (redox reactions) Are Common in Biological Systems ...
... Oxidation-Reduction Reactions (redox reactions) Are Common in Biological Systems ...
PDF(343KB)
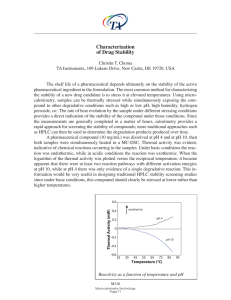
... rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time. A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-D ...
... rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time. A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-D ...
Aqueous chemistry is a very important component to laboratory
... 5. Add solvent until the solution reaches its final volume. The bottom of the meniscus should be on the flask mark. When making a solution using two liquids, follow the same steps, except in step 1, add the solute (in the liquid form) before adding the solvent in step 2. It is common to make up 1 co ...
... 5. Add solvent until the solution reaches its final volume. The bottom of the meniscus should be on the flask mark. When making a solution using two liquids, follow the same steps, except in step 1, add the solute (in the liquid form) before adding the solvent in step 2. It is common to make up 1 co ...
Honors Chemistry II Review 1. Express the following in scientific
... 15. A binary compound of zinc and sulfur contains 67.1% zinc by mass. What is the ratio of zinc and sulfur atoms in the compound? 16. Naturally occurring boron consists of two isotopes, 10B (19.9%), with an atomic mass of 10.0129, and 11B (80.1%) with an atomic mass of 11.00931. What is the atomic w ...
... 15. A binary compound of zinc and sulfur contains 67.1% zinc by mass. What is the ratio of zinc and sulfur atoms in the compound? 16. Naturally occurring boron consists of two isotopes, 10B (19.9%), with an atomic mass of 10.0129, and 11B (80.1%) with an atomic mass of 11.00931. What is the atomic w ...
1 - College of Arts and Sciences
... The states of the reactants and products are written in parentheses to the right of each compound Coefficients are inserted to balance the equation ...
... The states of the reactants and products are written in parentheses to the right of each compound Coefficients are inserted to balance the equation ...
1 - College of Arts and Sciences
... The states of the reactants and products are written in parentheses to the right of each compound Coefficients are inserted to balance the equation ...
... The states of the reactants and products are written in parentheses to the right of each compound Coefficients are inserted to balance the equation ...
Chapter 2 - Molecules of Life (Biochemistry) Periodic Table of
... Ions - The Octet Rule (Rule of Eight)! Atoms can gain or lose electrons! Except for the first electron shell, the outermost (valence) shell can hold 8 electrons (This applies to all atoms that you need to know about.)! E.g. Sodium atom (Na1123) loses one electron → Na+! • Giving something away is a ...
... Ions - The Octet Rule (Rule of Eight)! Atoms can gain or lose electrons! Except for the first electron shell, the outermost (valence) shell can hold 8 electrons (This applies to all atoms that you need to know about.)! E.g. Sodium atom (Na1123) loses one electron → Na+! • Giving something away is a ...
Atoms, Ions and Molecules
... In molecules, atoms share a pair of electrons to make chemical bonds with each other. Molecules made from only one kind of atom are molecules of an element. Molecules made from different kinds of a ...
... In molecules, atoms share a pair of electrons to make chemical bonds with each other. Molecules made from only one kind of atom are molecules of an element. Molecules made from different kinds of a ...
Document
... Calculate the atomic mass of iridium. Iridium has two isotopes. Iridium-191 has a mass of 191.0 amu and a percent abundance of 37.58%. Iridium-193 has a mass of 193.0 amu and a percent abundance of 62.42%. ...
... Calculate the atomic mass of iridium. Iridium has two isotopes. Iridium-191 has a mass of 191.0 amu and a percent abundance of 37.58%. Iridium-193 has a mass of 193.0 amu and a percent abundance of 62.42%. ...
Chemical Reactions are…
... Burning of wood results in products that appear to have less mass as ashes; where is the rest? Gases and smoke. ...
... Burning of wood results in products that appear to have less mass as ashes; where is the rest? Gases and smoke. ...
SAMPLE PAPER -2 Time Allowed: 3 Hrs
... (b) State Henry's law. Give its mathematical expression and write two applications of it. Q.29 (a) Account for the following: (i) Transition elements show highest oxidation state in their oxides than fluorides. (ii) Cu has positive electrode potential in the first transition series. (b)Write balance ...
... (b) State Henry's law. Give its mathematical expression and write two applications of it. Q.29 (a) Account for the following: (i) Transition elements show highest oxidation state in their oxides than fluorides. (ii) Cu has positive electrode potential in the first transition series. (b)Write balance ...
Paper - Edexcel
... reaction 1 ................... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ........................................ .............................................................................................. ...
... reaction 1 ................... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ........................................ .............................................................................................. ...
Sections 6.4 - 6.5
... Mg and Cu; in ship building as HYDRONALIUM, alloyed with 3-12 % Mg – with disastrous consequences in the BC SeaCat Ferry building program and the Falkland War: Al/Mg + n O2(g) → Al2O3 + MgO + lots of heat ! in water: Al/Mg + n H2O(l) → Al2O3 + MgO + n H2(g) ! …, i.e. Mg and Al burn underwater once i ...
... Mg and Cu; in ship building as HYDRONALIUM, alloyed with 3-12 % Mg – with disastrous consequences in the BC SeaCat Ferry building program and the Falkland War: Al/Mg + n O2(g) → Al2O3 + MgO + lots of heat ! in water: Al/Mg + n H2O(l) → Al2O3 + MgO + n H2(g) ! …, i.e. Mg and Al burn underwater once i ...
3 - Study Hungary
... A: the atomic number decreases by 2 and the mass number by 4. B: the atomic number decreases by 4 and the mass number by 2. C: the atomic number increases by 1 and the mass number doesn’t change. D: the loss of a neutron decreases the mass number by 1 and the charge by 1. E: the loss of a proton dec ...
... A: the atomic number decreases by 2 and the mass number by 4. B: the atomic number decreases by 4 and the mass number by 2. C: the atomic number increases by 1 and the mass number doesn’t change. D: the loss of a neutron decreases the mass number by 1 and the charge by 1. E: the loss of a proton dec ...
bonding notes for votech
... Bonding occurs to have complete outermost energy levels – to become like noble gases ...
... Bonding occurs to have complete outermost energy levels – to become like noble gases ...
Endothermic And Exothermic Reactions
... Chemical bonds and Energy Chemical energy is the energy stored in the chemical bonds of a substance. Energy changes in chemical reactions are determined by changes that occur in chemical bonding. Chemical reactions involve the breaking of bonds in the reactants and the formation of bonds in the pro ...
... Chemical bonds and Energy Chemical energy is the energy stored in the chemical bonds of a substance. Energy changes in chemical reactions are determined by changes that occur in chemical bonding. Chemical reactions involve the breaking of bonds in the reactants and the formation of bonds in the pro ...
PPTB&W - Gmu - George Mason University
... electrons make it a good electrical conductor Although Aluminum is a metal, its halides exist in the gaseous state as covalent dimers - AL2Cl6 (contrast salts of group 1 & 2 metals) Aluminum Oxide, Al2O3, is amphoteric (can act as an acid or base) rather than basic like the Group 1A & 2A metals Alth ...
... electrons make it a good electrical conductor Although Aluminum is a metal, its halides exist in the gaseous state as covalent dimers - AL2Cl6 (contrast salts of group 1 & 2 metals) Aluminum Oxide, Al2O3, is amphoteric (can act as an acid or base) rather than basic like the Group 1A & 2A metals Alth ...
chapter2 2012 (no naming) 2014
... • Sodium chloride (NaCl): Sodium cations and chloride anions associate into a continuous network ...
... • Sodium chloride (NaCl): Sodium cations and chloride anions associate into a continuous network ...
AS specification - word format File
... changes but also to detect drugs such as alcohol. The unit deals with issues regarding the environment, such as climate change, the effect of greenhouse gases, carbon footprints and other aspects of green chemistry. It ensures that students understand the underlying chemistry and can investigate way ...
... changes but also to detect drugs such as alcohol. The unit deals with issues regarding the environment, such as climate change, the effect of greenhouse gases, carbon footprints and other aspects of green chemistry. It ensures that students understand the underlying chemistry and can investigate way ...
6CH02 - MPPE
... Answer ALL the questions. Write your answers in the spaces provided. 17 This question is about the element chlorine and its compounds. (a) When chlorine is bubbled through water, a solution of chlorine water forms. What is the colour of chlorine water? ...
... Answer ALL the questions. Write your answers in the spaces provided. 17 This question is about the element chlorine and its compounds. (a) When chlorine is bubbled through water, a solution of chlorine water forms. What is the colour of chlorine water? ...
Mass-Mass Stoichiometry
... 38. How many moles are in a 45.0 L sample of O2 at STP? 39. What is the volume of a 0.77 mol sample of Ne gas at STP? a. How many Ne particles would this be? b. What would the mass of this sample be? 40. How many particles would there be in a 134 gram sample of HCl? 41. What is the pressure(in atm)e ...
... 38. How many moles are in a 45.0 L sample of O2 at STP? 39. What is the volume of a 0.77 mol sample of Ne gas at STP? a. How many Ne particles would this be? b. What would the mass of this sample be? 40. How many particles would there be in a 134 gram sample of HCl? 41. What is the pressure(in atm)e ...
Redox

Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. The term ""redox"" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as ""redox"" even though no electron transfer occurs (such as those involving covalent bonds).There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.