Availability and development of solid minerals in Cross River State
... The role of Government in solid minerals development is essential to provide conducive and enabling environment as well as required infrastructural support facilities. The development of the solid minerals industry has to be undertaken in a well-coordinated and organized manner in order to get the b ...
... The role of Government in solid minerals development is essential to provide conducive and enabling environment as well as required infrastructural support facilities. The development of the solid minerals industry has to be undertaken in a well-coordinated and organized manner in order to get the b ...
1 - SUrface
... Despite crystallization from THF/toluene mixtures neither of the reaction products contains coordinated THF molecules, indicating that extensive K-C coordination maybe advantageous over a small number of K-THF interaction. A similar phenomenon was recently observed with [Ba(6-Ph)2B(Ph)2(thf)3][BPh ...
... Despite crystallization from THF/toluene mixtures neither of the reaction products contains coordinated THF molecules, indicating that extensive K-C coordination maybe advantageous over a small number of K-THF interaction. A similar phenomenon was recently observed with [Ba(6-Ph)2B(Ph)2(thf)3][BPh ...
CHEM 101 Final (Term 151)
... 34. Which one of the following statements is TRUE? A) The magnetic quantum number (ml) describes the orientation of an orbital. B) The principal quantum number (n) describes the shape of an orbital. C) The principal quantum number (n) describes the orientation of an orbital. D) The angular momentum ...
... 34. Which one of the following statements is TRUE? A) The magnetic quantum number (ml) describes the orientation of an orbital. B) The principal quantum number (n) describes the shape of an orbital. C) The principal quantum number (n) describes the orientation of an orbital. D) The angular momentum ...
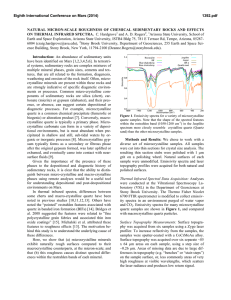
Natural Micron-Scale Roughness of Chemical Sedimentary Rocks
... biogenic) or alteration product [7]. Conversely, macrocrystalline quartz is typically a primary phase. Microcrystalline carbonate can form in a variety of depositional environments, but is most abundant when precipitated in shallow and still, sub-tidal waters by organic or inorganic processes [8]. M ...
... biogenic) or alteration product [7]. Conversely, macrocrystalline quartz is typically a primary phase. Microcrystalline carbonate can form in a variety of depositional environments, but is most abundant when precipitated in shallow and still, sub-tidal waters by organic or inorganic processes [8]. M ...
Course Packs
... • Scientists start with a seed, which is a small piece of natural crystal. Some of the advantages of tissue culture include: ...
... • Scientists start with a seed, which is a small piece of natural crystal. Some of the advantages of tissue culture include: ...
1. some basic concepts of chemistry
... Every experimental measurement has some amount of uncertainty associated with it. The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty. The uncertainty is in ...
... Every experimental measurement has some amount of uncertainty associated with it. The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty. The uncertainty is in ...
Mineral
... is it a mineral too? Formed by natural process – occurs on or inside earth Has a definite chemical composition Orderly arrangement of atoms in a repeated pattern forming crystals. Inorganic Solid Coal is formed by plant and animal material (ORGANIC) breaking down and with pressure over time ...
... is it a mineral too? Formed by natural process – occurs on or inside earth Has a definite chemical composition Orderly arrangement of atoms in a repeated pattern forming crystals. Inorganic Solid Coal is formed by plant and animal material (ORGANIC) breaking down and with pressure over time ...
AP Chemistry Summer Assignment
... 10. A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether soli ...
... 10. A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether soli ...
PESUnit5minerals12-13
... • Crystallization – In hot magma, atoms are not bound in crystal form. As magma cools the atoms arrange themselves into crystalline structures forming minerals! – Happens underground or at the surface in volcanically active places ...
... • Crystallization – In hot magma, atoms are not bound in crystal form. As magma cools the atoms arrange themselves into crystalline structures forming minerals! – Happens underground or at the surface in volcanically active places ...
Experimental determination of hydromagnesite precipitation rates
... However, there is still no universally applicable solution to mitigate silica precipitation because our understanding of the pathways and mechanisms of silica precipitation under conditions prevalent in geothermal power plants (i.e. high temperatures and high flow rates) is still in its infancy. Her ...
... However, there is still no universally applicable solution to mitigate silica precipitation because our understanding of the pathways and mechanisms of silica precipitation under conditions prevalent in geothermal power plants (i.e. high temperatures and high flow rates) is still in its infancy. Her ...
Complex Ions and Free Energy
... 2. A solution containing 0.010 M Ba2+ and 0.10 M Ag+, which solid will precipitate first when Na2SO4 is added to the solution? Justify your reasoning with calculations. NOTE the Ksp (BaSO4) = 1.1 x 10-10 and Ksp (AgSO4) = 1.1 x 10-5. ...
... 2. A solution containing 0.010 M Ba2+ and 0.10 M Ag+, which solid will precipitate first when Na2SO4 is added to the solution? Justify your reasoning with calculations. NOTE the Ksp (BaSO4) = 1.1 x 10-10 and Ksp (AgSO4) = 1.1 x 10-5. ...
CP Chemistry Practice Mid
... 13. When gases are heated, their kinetic energy a. increases b. decreases c. remains the same ...
... 13. When gases are heated, their kinetic energy a. increases b. decreases c. remains the same ...
2004 NEACS Ashdown Exam 1. The allotrope of carbon shown to
... (C) A sample contains many molecules, each of which has one C O bond shorter than the other two. Averaging all of these molecules produces an average distance that is somewhat shorter that a single C O bond. (D) One C O bond is more reactive than the other two because the double bond is less stable. ...
... (C) A sample contains many molecules, each of which has one C O bond shorter than the other two. Averaging all of these molecules produces an average distance that is somewhat shorter that a single C O bond. (D) One C O bond is more reactive than the other two because the double bond is less stable. ...
Rocks
... - a solid, naturally-occurring, inorganic element or compound with defined chemical composition. They are also crystalline in structure. Based on regular arrangement of atoms or ions within it. ...
... - a solid, naturally-occurring, inorganic element or compound with defined chemical composition. They are also crystalline in structure. Based on regular arrangement of atoms or ions within it. ...
7.5.9 Compare physical properties of matter to the chemical property
... 7.5.9 Compare physical properties of matter to the chemical property of reactivity with a certain substance ...
... 7.5.9 Compare physical properties of matter to the chemical property of reactivity with a certain substance ...
Density of solutions answers The concentration of solutions is often
... A compound containing only carbon and hydrogen is analyzed, and is found to contain 74.88% carbon on a mass basis. Show how the empirical formula of the compound may be calculated. If 1.000g of elemental carbon is heated in a stream of hydrogen gas, 1.335g of a compound containing only carbon and h ...
... A compound containing only carbon and hydrogen is analyzed, and is found to contain 74.88% carbon on a mass basis. Show how the empirical formula of the compound may be calculated. If 1.000g of elemental carbon is heated in a stream of hydrogen gas, 1.335g of a compound containing only carbon and h ...
KEY 1. An ATOM is the smallest particle into which an element can
... 25. Gold, silver, and copper are all examples of NATIVE ELEMENTS, or minerals which exists as single chemical elements. 26. The atomic number of an element is equal to the number of A. protons in the nucleus B. neutrons in the nucleus C. electrons swirling around the nucleus D. protons plus neutrons ...
... 25. Gold, silver, and copper are all examples of NATIVE ELEMENTS, or minerals which exists as single chemical elements. 26. The atomic number of an element is equal to the number of A. protons in the nucleus B. neutrons in the nucleus C. electrons swirling around the nucleus D. protons plus neutrons ...
Physical Properties of the Gemstones
... specific gravity. Heavier gemstones are usually harder as well. The range is from amber, which has a specific gravity of 1.08 and opal, with a specific gravity of 2.05, all the way up to corundum (sapphires and rubies) with a specific gravity of 3.99, spessartite garnet, specific gravity of 4.15, ma ...
... specific gravity. Heavier gemstones are usually harder as well. The range is from amber, which has a specific gravity of 1.08 and opal, with a specific gravity of 2.05, all the way up to corundum (sapphires and rubies) with a specific gravity of 3.99, spessartite garnet, specific gravity of 4.15, ma ...
chemistry 11 exam review
... 8. What pressure is needed to change 130 mL of gas at 740 torr to 150 mL? (641 torr) 9. What temperature change is needed to change 1.0 L of gas at 10.0C and 800.0 torr to 0.50 L and 760 torr? (-138C change to bring your final temperature to 134K) 10. A 1.0 L rubber bladder is filled with carbon d ...
... 8. What pressure is needed to change 130 mL of gas at 740 torr to 150 mL? (641 torr) 9. What temperature change is needed to change 1.0 L of gas at 10.0C and 800.0 torr to 0.50 L and 760 torr? (-138C change to bring your final temperature to 134K) 10. A 1.0 L rubber bladder is filled with carbon d ...
LATENT HEAT AND ELECTRODE POTENTIAL
... come greater than X. Such results are contrary both to experience and to the principles of thermodynamics. Theory (7) and experiment indicate that at the mp of M, the liquid and solid forms of M must have the same potential. Although this potential, or the emf, E, of a cell of which M forms one elec ...
... come greater than X. Such results are contrary both to experience and to the principles of thermodynamics. Theory (7) and experiment indicate that at the mp of M, the liquid and solid forms of M must have the same potential. Although this potential, or the emf, E, of a cell of which M forms one elec ...
Unit 5 - PLANET EARTH TOPIC 1 – MINERALS
... 1. Mineral: A pure, naturally occurring, non-living crystalline material 2. Crust: The outermost layer of Earth 3. Rock: Is made up of more than one mineral 4. Element: A pure substance 5. Compound: Made up of two or more elements combined 2. In order to survive your body needs 20 or more different ...
... 1. Mineral: A pure, naturally occurring, non-living crystalline material 2. Crust: The outermost layer of Earth 3. Rock: Is made up of more than one mineral 4. Element: A pure substance 5. Compound: Made up of two or more elements combined 2. In order to survive your body needs 20 or more different ...
Inquiry: Calculation - Coristines
... 4. Using methane gas as an example, explain why one can not assume the heat of combustion is the reverse of the heat of formation (3 marks) 5. Determine the complete combustion of ethanoic acid using bond energies and heats of formation (assume reaction occurs at SATP). Compare your results for each ...
... 4. Using methane gas as an example, explain why one can not assume the heat of combustion is the reverse of the heat of formation (3 marks) 5. Determine the complete combustion of ethanoic acid using bond energies and heats of formation (assume reaction occurs at SATP). Compare your results for each ...
Spring 2009 Final Exam Review – Part 2
... Covalent Bonds – a strong bond that occurs through the sharing of electrons between 2 nonmetals. You can draw Lewis structures for covalent molecules. Determine the total # of valence electrons in the molecule. Divide that number by 2 to determine the number of pairs of electrons available to ...
... Covalent Bonds – a strong bond that occurs through the sharing of electrons between 2 nonmetals. You can draw Lewis structures for covalent molecules. Determine the total # of valence electrons in the molecule. Divide that number by 2 to determine the number of pairs of electrons available to ...
chemistry - ALLEN Jaipur
... of the solution. How is molar conductivity of a solute related to conductivity of its solution? (b) A voltaic cell is set up at 25° C. with the following half-cells: Al |Ag3+ (0.001 M) and Ni|Ni2+ (0.50 M) Calculate the cell voltage [ ...
... of the solution. How is molar conductivity of a solute related to conductivity of its solution? (b) A voltaic cell is set up at 25° C. with the following half-cells: Al |Ag3+ (0.001 M) and Ni|Ni2+ (0.50 M) Calculate the cell voltage [ ...
Reactions in Aqueous Solution (Brown 13th-Fossum
... • We have to know the mass (and, therefore, number of moles) of the solute. • The solute is added to a volumetric flask. Don’t ever go beyond the calibration mark… Or you’ll have to start all over again... Dilution • Dilution: Lowering concentration by adding additional solvent. • Dilution factor: T ...
... • We have to know the mass (and, therefore, number of moles) of the solute. • The solute is added to a volumetric flask. Don’t ever go beyond the calibration mark… Or you’ll have to start all over again... Dilution • Dilution: Lowering concentration by adding additional solvent. • Dilution factor: T ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.