AP Chemistry Summer Assignment 2016
... AP Chemistry: Solubility Rules The following solubility rules are guidelines for determining an ionic compound's solubility, or its ability to dissolve in water. Note that nothing is completely insoluble in water. For each family of ions, the rule and the exception ions are given. Note that mercury ...
... AP Chemistry: Solubility Rules The following solubility rules are guidelines for determining an ionic compound's solubility, or its ability to dissolve in water. Note that nothing is completely insoluble in water. For each family of ions, the rule and the exception ions are given. Note that mercury ...
Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
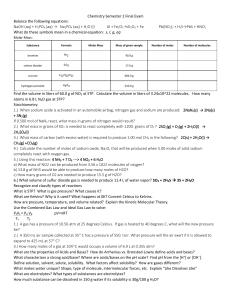
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
Advanced Placement Chemistry Test
... In a saturated solution of MgF2 at 18° C, the concentration of Mg2+ is 1.21 x 10¯3 molar. The equilibrium is represented by the equation above. (a) ...
... In a saturated solution of MgF2 at 18° C, the concentration of Mg2+ is 1.21 x 10¯3 molar. The equilibrium is represented by the equation above. (a) ...
Chemistry II Exams and Keys 2013 Season
... A. Diammonium iron(II) sulfate hydrate B. Ammonium iron(II) sulfate hydrate C. Ammonium iron(II) disulfate hexahydrate D. Ammonium iron(III) sulfate hexahydrate E. Ammonium iron(II) sulfate hexahydrate 13. The figure depicts the unit cell for a compound containing atoms X (filled circles) and Z (ope ...
... A. Diammonium iron(II) sulfate hydrate B. Ammonium iron(II) sulfate hydrate C. Ammonium iron(II) disulfate hexahydrate D. Ammonium iron(III) sulfate hexahydrate E. Ammonium iron(II) sulfate hexahydrate 13. The figure depicts the unit cell for a compound containing atoms X (filled circles) and Z (ope ...
Bellin College Homework Supplement
... lowering the body temperature will reduce the amount of oxygen needed by the body. Some methods used to lower body temperature include cooled saline solution, cool water blankets, or cooling caps worn on the head. How many kilojoules are lost when the body temperature of a surgery patient with a blo ...
... lowering the body temperature will reduce the amount of oxygen needed by the body. Some methods used to lower body temperature include cooled saline solution, cool water blankets, or cooling caps worn on the head. How many kilojoules are lost when the body temperature of a surgery patient with a blo ...
Hardness Cleavage Fracture Luster Color Specific Gravity / Density
... sample is weak for one reason or another, but still, hardness is something easily investigated. Check out the Moh’s Scale of Relative Hardness in this brochure. Minerals are listed from the softest, #1 to the hardest, #10. You can figure out the hardness of a sample by trying to scratch it with know ...
... sample is weak for one reason or another, but still, hardness is something easily investigated. Check out the Moh’s Scale of Relative Hardness in this brochure. Minerals are listed from the softest, #1 to the hardest, #10. You can figure out the hardness of a sample by trying to scratch it with know ...
Ch 1 Earth Materials
... • Explain the different kinds of bonds and describe their influence on mineral characteristics • Define and distinguish between minerals and rocks • List key properties used to identify minerals • Identify most common mineral families and accessory mineral families • Explain what holds rocks togethe ...
... • Explain the different kinds of bonds and describe their influence on mineral characteristics • Define and distinguish between minerals and rocks • List key properties used to identify minerals • Identify most common mineral families and accessory mineral families • Explain what holds rocks togethe ...
Effects of polymorphism on charge transport in
... single-crystal field-effect transistors used in this study is presented in Fig. 1共b兲. The source and drain Ti/Au electrodes 共5 nm Ti, 40 nm Au兲 were deposited by e-beam evaporation and patterned by photolithography and a lift-off process. The clean substrates were immersed in an 8 mol/l solution of ...
... single-crystal field-effect transistors used in this study is presented in Fig. 1共b兲. The source and drain Ti/Au electrodes 共5 nm Ti, 40 nm Au兲 were deposited by e-beam evaporation and patterned by photolithography and a lift-off process. The clean substrates were immersed in an 8 mol/l solution of ...
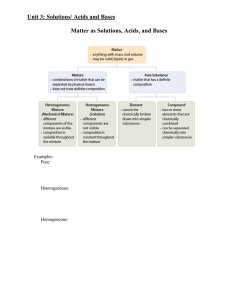
Chapter 4: Solution Chemistry and the Hydrosphere
... • How can a solution hold more solute than it should be able to hold? – If a given amount of solute is dissolved in a solvent at a higher temperature, and the solution is allowed to cool without being disturbed, the solute will remain in solution. • But the solution is unstable, and the solute will ...
... • How can a solution hold more solute than it should be able to hold? – If a given amount of solute is dissolved in a solvent at a higher temperature, and the solution is allowed to cool without being disturbed, the solute will remain in solution. • But the solution is unstable, and the solute will ...
B. The Physical Properties of Matter
... suitable solvent. The solvent is allowed to slowly evaporate causing some of the desired solid to come out of solution as crystals; however, not all the solvent is allowed to evaporate. The crystals that are formed can then be separated by HAND SEPARATION or FILTRATION. ...
... suitable solvent. The solvent is allowed to slowly evaporate causing some of the desired solid to come out of solution as crystals; however, not all the solvent is allowed to evaporate. The crystals that are formed can then be separated by HAND SEPARATION or FILTRATION. ...
The Physical Properties And Physical Changes of Substances
... suitable solvent. The solvent is allowed to slowly evaporate causing some of the desired solid to come out of solution as crystals; however, not all the solvent is allowed to evaporate. The crystals that are formed can then be separated by HAND SEPARATION or FILTRATION. ...
... suitable solvent. The solvent is allowed to slowly evaporate causing some of the desired solid to come out of solution as crystals; however, not all the solvent is allowed to evaporate. The crystals that are formed can then be separated by HAND SEPARATION or FILTRATION. ...
Chapter 4 Mesozoic Sedimentary and Volcanic Rocks
... rhyolite lavas generally contain euhedral phenocrysts of quartz and feldspar, but some of these crystals have been mechanically broken as a result of fragmentation during eruption. Banded porphyritic rhyolite lava is exposed on the summit of Por Kai Shan (1398 1633) and on the south side of the hill ...
... rhyolite lavas generally contain euhedral phenocrysts of quartz and feldspar, but some of these crystals have been mechanically broken as a result of fragmentation during eruption. Banded porphyritic rhyolite lava is exposed on the summit of Por Kai Shan (1398 1633) and on the south side of the hill ...
Past AP FRQ`s Linked to Text Chapters
... In an experiment to determine the molecular weight and the ionization constant for ascorbic acid (vitamin C), a student dissolved 1.3717 grams of the acid in water to make 50.00 milliliters of solution. The entire solution was titrated with a 0.2211molar NaOH solution. The pH was monitored throughou ...
... In an experiment to determine the molecular weight and the ionization constant for ascorbic acid (vitamin C), a student dissolved 1.3717 grams of the acid in water to make 50.00 milliliters of solution. The entire solution was titrated with a 0.2211molar NaOH solution. The pH was monitored throughou ...
Mineral power point talk
... form minerals. If the magma cools quickly the crystals will be small crystals. If the magma cools slowly the crystals will be large. The second way minerals form is from solutions. A solution can be supersaturated, which means the solution is holding all the dissolved molecules it can hold. Minerals ...
... form minerals. If the magma cools quickly the crystals will be small crystals. If the magma cools slowly the crystals will be large. The second way minerals form is from solutions. A solution can be supersaturated, which means the solution is holding all the dissolved molecules it can hold. Minerals ...
Outline for Unit 1 Solutions, Acid/Base, and Gases
... 3. Temperature – at higher temp kinetic energy of the solvent is higher so more collisions of solvent molecules with solute 4. Particle size – smaller particles dissolve faster since there is more surface area available to solvent 5. Pressure (partial pressure) – only affects gas in liquids – solubi ...
... 3. Temperature – at higher temp kinetic energy of the solvent is higher so more collisions of solvent molecules with solute 4. Particle size – smaller particles dissolve faster since there is more surface area available to solvent 5. Pressure (partial pressure) – only affects gas in liquids – solubi ...
makeup6
... 15. The Ksp of PbBr2 is 6.3 x 10¯6. If 50 mL of 0.020 M Pb(NO3)2 are mixed with 50 mL of 0.010 M CaBr2, which of the following is true? (A) the solution will not form a precipitate (B) calcium nitrate will precipitate (C) PbBr2 will precipitate and excess Pb2+ will remain in solution (D) PbBr2 will ...
... 15. The Ksp of PbBr2 is 6.3 x 10¯6. If 50 mL of 0.020 M Pb(NO3)2 are mixed with 50 mL of 0.010 M CaBr2, which of the following is true? (A) the solution will not form a precipitate (B) calcium nitrate will precipitate (C) PbBr2 will precipitate and excess Pb2+ will remain in solution (D) PbBr2 will ...
Minerals - Geology
... There are 88 naturally occurring elements in Earth’s crust. There are 92 naturally occurring elements all together on Earth. ...
... There are 88 naturally occurring elements in Earth’s crust. There are 92 naturally occurring elements all together on Earth. ...
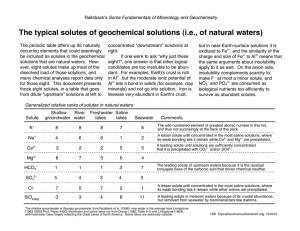
The typical solutes of geochemical solutions (i.e., of natural waters)
... The typical solutes of geochemical solutions (i.e., of natural waters) The periodic table offers up 92 naturally occurring elements that could seemingly be included as solutes in the geochemical solutions that are natural waters. However, eight solutes make up most of the dissolved load of those sol ...
... The typical solutes of geochemical solutions (i.e., of natural waters) The periodic table offers up 92 naturally occurring elements that could seemingly be included as solutes in the geochemical solutions that are natural waters. However, eight solutes make up most of the dissolved load of those sol ...
Solutes
... 2. 50 g of KCl is dissolved in 100 g of water at 50°C. Is the solution saturated, unsaturated or supersaturated? ...
... 2. 50 g of KCl is dissolved in 100 g of water at 50°C. Is the solution saturated, unsaturated or supersaturated? ...
Name ionic compounds containing main group or
... A chemist has a 100-gram sample of a compound that contains 17.073 grams of carbon, 2.168 grams of hydrogen, 10.840 grams of oxygen, 8.5366 grams of nitrogen, 28.8618 grams of chlorine and the rest is bromine. What is the empirical formula of the compound? Refer to Question # 27 to answer this quest ...
... A chemist has a 100-gram sample of a compound that contains 17.073 grams of carbon, 2.168 grams of hydrogen, 10.840 grams of oxygen, 8.5366 grams of nitrogen, 28.8618 grams of chlorine and the rest is bromine. What is the empirical formula of the compound? Refer to Question # 27 to answer this quest ...
minerals - Ms. Sheehans Geology Class
... ___________of a gas or liquid come together to form a solid mineral. 1. The atoms are arranged in an ordered three-dimensional array that is ________________ in all directions. 2. The crystal _________ as more atoms are added onto the structure. B. Minerals can form from a _____________ as it begins ...
... ___________of a gas or liquid come together to form a solid mineral. 1. The atoms are arranged in an ordered three-dimensional array that is ________________ in all directions. 2. The crystal _________ as more atoms are added onto the structure. B. Minerals can form from a _____________ as it begins ...
Spring 2002 - Kwantlen Polytechnic University
... b. The solution shows a positive deviation from Raoult’s Law. c. The solution shows a negative deviation from Raoult’s Law and possesses a minimum boiling point azeotrope. d. The solution shows a negative deviation from Raoult’s Law and possesses a maximum boiling point azeorope. e. The solution pro ...
... b. The solution shows a positive deviation from Raoult’s Law. c. The solution shows a negative deviation from Raoult’s Law and possesses a minimum boiling point azeotrope. d. The solution shows a negative deviation from Raoult’s Law and possesses a maximum boiling point azeorope. e. The solution pro ...
Chemistry Final Exam Review 2006-2007
... pressure does not change? d) The volume of a sample of oxygen gas is 300.0 ml when the pressure is 1.00 atm and the temperature is 27.0 C. At what temperature would the volume change to 1.00 L and the pressure change to 0.500 atm? e) A sample of gas at 25.0 C has a volume of 11.0 L and exerts a pres ...
... pressure does not change? d) The volume of a sample of oxygen gas is 300.0 ml when the pressure is 1.00 atm and the temperature is 27.0 C. At what temperature would the volume change to 1.00 L and the pressure change to 0.500 atm? e) A sample of gas at 25.0 C has a volume of 11.0 L and exerts a pres ...
The Urinary F1 Activation Peptide of Human
... is the predominant protein incorporated into calcium oxalate crystals precipitated from human urine. The aim of this study was to examine the effect of pure urinary prothrombin F1 on calcium oxalate crystallization in human urine. 2. Urinary prothrombin F1 was purified from demineralized calcium oxa ...
... is the predominant protein incorporated into calcium oxalate crystals precipitated from human urine. The aim of this study was to examine the effect of pure urinary prothrombin F1 on calcium oxalate crystallization in human urine. 2. Urinary prothrombin F1 was purified from demineralized calcium oxa ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.