SOLLIQSOL questions
... (b) Describe any changes that can be observed in a sample of solid argon when the temperature is increased from 40 K to 160 K at a constant pressure of 0.50 atmosphere. (c) Describe any changes that can be observed in a sample of liquid argon when the pressure is reduced from 10 atmospheres to 1 atm ...
... (b) Describe any changes that can be observed in a sample of solid argon when the temperature is increased from 40 K to 160 K at a constant pressure of 0.50 atmosphere. (c) Describe any changes that can be observed in a sample of liquid argon when the pressure is reduced from 10 atmospheres to 1 atm ...
inorganic chemistry
... This explores the trend in oxidising ability of the Group 7 elements (the halogens) fluorine, chlorine, bromine and iodine. We are going to look at the ability of one halogen to oxidise the ions of another one, and how that changes as you go down the Group. We are going to look at the reactions betw ...
... This explores the trend in oxidising ability of the Group 7 elements (the halogens) fluorine, chlorine, bromine and iodine. We are going to look at the ability of one halogen to oxidise the ions of another one, and how that changes as you go down the Group. We are going to look at the reactions betw ...
Nafion® Dryers and Humidifiers The Ten Most Common Questions
... wall. Drying/humidification stops when there is no longer a gradient; at this point equilibrium has been reached. It might seem that if the water vapor pressure outside the tubing were zero, the water vapor pressure of the sample inside the tubing would eventually fall to zero also. This is unfortun ...
... wall. Drying/humidification stops when there is no longer a gradient; at this point equilibrium has been reached. It might seem that if the water vapor pressure outside the tubing were zero, the water vapor pressure of the sample inside the tubing would eventually fall to zero also. This is unfortun ...
File
... (b) Enzymes are particular types of proteins that catalyse chemical reactions. The efficiency of enzymes can be reduced by the presence of other molecules known as inhibitors. Explain how both competitive and non-competitive inhibitors prevent enzymes from ...
... (b) Enzymes are particular types of proteins that catalyse chemical reactions. The efficiency of enzymes can be reduced by the presence of other molecules known as inhibitors. Explain how both competitive and non-competitive inhibitors prevent enzymes from ...
Computational Study of protonation of ozone
... As can be seen from Fig. 1, all forms of protonated ozone singlet electron configuration are flat. In the first two cases, in the proton is trans- (structure 1a) and cis- (structure 1b) position with O-O bond of ozone. Oxygen-oxygen bonds in ozone are unequal. Calculated dipole moment of ozone is 0. ...
... As can be seen from Fig. 1, all forms of protonated ozone singlet electron configuration are flat. In the first two cases, in the proton is trans- (structure 1a) and cis- (structure 1b) position with O-O bond of ozone. Oxygen-oxygen bonds in ozone are unequal. Calculated dipole moment of ozone is 0. ...
Post Lab Questions
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
Elements, Compounds, and Chemical Equations
... Count the atoms in the reactants and the products. • Count the total number of each type of atom on the reactant (ingredient) side. • Count the total number of each type of atom in the product (what you make) side • If the number of each type of atom matches, the equation is balanced. If the numbers ...
... Count the atoms in the reactants and the products. • Count the total number of each type of atom on the reactant (ingredient) side. • Count the total number of each type of atom in the product (what you make) side • If the number of each type of atom matches, the equation is balanced. If the numbers ...
SELECTED ANSWERS
... 35. Same answer as problem 33 but with sodium ions, Na+, instead of lithium ions, Li+, and sulfate ions, SO42-, in the place of iodide ions, I-. The final solution will have two times as many sodium ions as sulfate ions. 37. In a solution of a solid in a liquid, the solid is generally considered the ...
... 35. Same answer as problem 33 but with sodium ions, Na+, instead of lithium ions, Li+, and sulfate ions, SO42-, in the place of iodide ions, I-. The final solution will have two times as many sodium ions as sulfate ions. 37. In a solution of a solid in a liquid, the solid is generally considered the ...
H 2 O
... Ionization: H2O H2O+ + e— having a excess kinetic energy Excitation : H2O H2O*. This process is minor w/ ionization ...
... Ionization: H2O H2O+ + e— having a excess kinetic energy Excitation : H2O H2O*. This process is minor w/ ionization ...
COMPLEX IONS AND AMPHOTERISM
... metal hydroxide precipitate in each case.) In the case of the bismuth(III) and antimony(III) solutions, add enough 6 M NaOH to neutralize the HCl you added to dissolve the oxychloride and then add at least 1-3 drops more. Use your litmus paper to make sure the solution is basic at this point (red li ...
... metal hydroxide precipitate in each case.) In the case of the bismuth(III) and antimony(III) solutions, add enough 6 M NaOH to neutralize the HCl you added to dissolve the oxychloride and then add at least 1-3 drops more. Use your litmus paper to make sure the solution is basic at this point (red li ...
Document
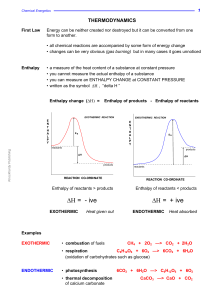
... changes in combustion reactions the inner metal chamber or bomb contains the sample and pure oxygen an electric coil ignites the sample temperature changes in the water surrounding the inner “bomb” are used to calculate ΔH ...
... changes in combustion reactions the inner metal chamber or bomb contains the sample and pure oxygen an electric coil ignites the sample temperature changes in the water surrounding the inner “bomb” are used to calculate ΔH ...
Chemistry
... sketch and construct cells not composed of metals and solutions of their ions including cells with inert electrodes and electrolytes containing species with different oxidation states ...
... sketch and construct cells not composed of metals and solutions of their ions including cells with inert electrodes and electrolytes containing species with different oxidation states ...
Chemistry
... (a) explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium (b) state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in concentration, pressure or temperature, ...
... (a) explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium (b) state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in concentration, pressure or temperature, ...
File - Science with Mr. Louie
... 12.257 (5 total significant figures) x 1.162 (4 total significant figures) 14.2426 rounds off to 14.24 (4 total significant figures) As a general rule, if you are unsure how many significant figures to us on the AP exam, use 3 significant figures. This may not always work but it will work most tim ...
... 12.257 (5 total significant figures) x 1.162 (4 total significant figures) 14.2426 rounds off to 14.24 (4 total significant figures) As a general rule, if you are unsure how many significant figures to us on the AP exam, use 3 significant figures. This may not always work but it will work most tim ...
Chemistry
... cresols. Ethers – simple and mixed, nomenclature of alcohols, phenols, ethers. Preparation of alcohols: by acid catalysed hydration of alkene, general reaction and examples, by hydroboration-oxidation of propene, from carbonyl compounds: hydrogenation of aldehydes, ketones, reduction of carboxylic a ...
... cresols. Ethers – simple and mixed, nomenclature of alcohols, phenols, ethers. Preparation of alcohols: by acid catalysed hydration of alkene, general reaction and examples, by hydroboration-oxidation of propene, from carbonyl compounds: hydrogenation of aldehydes, ketones, reduction of carboxylic a ...
Electrical conductivity of polycrystalline Mg(OH)
... separates mineral surfaces in polycrystalline aggregates where thin films of water of variable thickness can be formed. For instance, the film thickness reaches 85–185 nm in the case of a (111) NaCl surface in contact with a CaF2 (111) face under a contact pressure of a few MPa at room temperature ( ...
... separates mineral surfaces in polycrystalline aggregates where thin films of water of variable thickness can be formed. For instance, the film thickness reaches 85–185 nm in the case of a (111) NaCl surface in contact with a CaF2 (111) face under a contact pressure of a few MPa at room temperature ( ...
Titration #1 Determination of [NaOH]
... Three sentences at most: Purpose; general method; results. The brief introduction on the other side of this sheet may be helpful. {C, 3} ...
... Three sentences at most: Purpose; general method; results. The brief introduction on the other side of this sheet may be helpful. {C, 3} ...
Η - Knockhardy
... Further readings are taken every half minute as the reaction mixture cools. Extrapolate the lines as shown and calculate the value of ∆Τ. ...
... Further readings are taken every half minute as the reaction mixture cools. Extrapolate the lines as shown and calculate the value of ∆Τ. ...
Feasibility Study of using FAIMS to Detect Carbonyl Sulfide in Propane
... Sample preparation and introduction FAIMS can be used to detect volatiles in aqueous, solid and gaseous matrices and can consequently be used for a wide variety of applications. The user requirements and sample matrix for each application define the sample preparation and introduction steps required ...
... Sample preparation and introduction FAIMS can be used to detect volatiles in aqueous, solid and gaseous matrices and can consequently be used for a wide variety of applications. The user requirements and sample matrix for each application define the sample preparation and introduction steps required ...
Part II - American Chemical Society
... iii. (1) N is more electronegative that P, so electron density is shifted from H atoms towards the N, so the H+ can be more readily removed. (2) NO3– is stabilized by resonance more than H2PO4–. (3) HNO3 has two free oxygen atoms that attract electron density from the H atom, whereas H3PO4 has only ...
... iii. (1) N is more electronegative that P, so electron density is shifted from H atoms towards the N, so the H+ can be more readily removed. (2) NO3– is stabilized by resonance more than H2PO4–. (3) HNO3 has two free oxygen atoms that attract electron density from the H atom, whereas H3PO4 has only ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.




















![Titration #1 Determination of [NaOH]](http://s1.studyres.com/store/data/006790962_1-275eb40711c8f3ecc850bd432b1a775b-300x300.png)