ch22_lecture_6e_final
... The Nitrogen Cycle • The nitrogen cycle involves a direct interaction between land and sea. • Atmospheric N2 must be fixed to enter the land and sea. – Atmospheric fixation occurs when lightning provides the energy for the reaction between N2(g) and O2(g). – Industrial fixation results from the pro ...
... The Nitrogen Cycle • The nitrogen cycle involves a direct interaction between land and sea. • Atmospheric N2 must be fixed to enter the land and sea. – Atmospheric fixation occurs when lightning provides the energy for the reaction between N2(g) and O2(g). – Industrial fixation results from the pro ...
Chemical Changes and Structure Homework Booklet
... 12Mg are two different kinds of magnesium atom. a. What word is used to describe these types of atoms? b. Explain why they can be regarded as atoms of the same element? c. The relative atomic mass of magnesium is 24.3. What does this tell you about the relative amounts of each atom? An atom has atom ...
... 12Mg are two different kinds of magnesium atom. a. What word is used to describe these types of atoms? b. Explain why they can be regarded as atoms of the same element? c. The relative atomic mass of magnesium is 24.3. What does this tell you about the relative amounts of each atom? An atom has atom ...
E/F Physical Science
... 10. Circle the letter of the correct answer. If one carbon atom has an atomic mass of 12.0 amu and one oxygen atom has an atomic mass of 16.0 amu, what is the molar mass of carbon dioxide? a. 28.0 amu ...
... 10. Circle the letter of the correct answer. If one carbon atom has an atomic mass of 12.0 amu and one oxygen atom has an atomic mass of 16.0 amu, what is the molar mass of carbon dioxide? a. 28.0 amu ...
chapter 2
... b. funnel: to put solids or liquids into another container. c. graduated cylinder: to measure precise volumes of liquids (mL) d. balance: measures mass (g) e. stirring rod – used to stir solutions f. ruler – used to measure length (cm) ...
... b. funnel: to put solids or liquids into another container. c. graduated cylinder: to measure precise volumes of liquids (mL) d. balance: measures mass (g) e. stirring rod – used to stir solutions f. ruler – used to measure length (cm) ...
No Slide Title
... water, results in a solution that can conduct small electricity A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. ...
... water, results in a solution that can conduct small electricity A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. ...
2007 - SAASTA
... the container. Solids retain both their shape and volume when moved from one container ...
... the container. Solids retain both their shape and volume when moved from one container ...
unit 7 – writing and balancing chemical equations
... metals shown below. It is not necessary to memorize it because it will be provided for you on all quizzes and exams, but you must know how to use it AND YOU MUST REMEMBER TO USE IT! ACTIVITY SERIES ...
... metals shown below. It is not necessary to memorize it because it will be provided for you on all quizzes and exams, but you must know how to use it AND YOU MUST REMEMBER TO USE IT! ACTIVITY SERIES ...
AP Chemistry Summer Assignment
... substance, B, and a gas, C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solids A and B and the gas C are elements or compounds? Explain your conclusions for each substance. ...
... substance, B, and a gas, C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solids A and B and the gas C are elements or compounds? Explain your conclusions for each substance. ...
water - Portal UniMAP
... The strength of an acis is expressed as its pK. Henderson-Hasselbalch equation relates the pH of a solution to the pK and concentration of an acid to its conjugate base. Buffered solutions resist changes in pH within about one pH unit of the pK of the buffering ...
... The strength of an acis is expressed as its pK. Henderson-Hasselbalch equation relates the pH of a solution to the pK and concentration of an acid to its conjugate base. Buffered solutions resist changes in pH within about one pH unit of the pK of the buffering ...
water - Portal UniMAP
... The strength of an acis is expressed as its pK. Henderson-Hasselbalch equation relates the pH of a solution to the pK and concentration of an acid to its conjugate base. Buffered solutions resist changes in pH within about one pH unit of the pK of the buffering ...
... The strength of an acis is expressed as its pK. Henderson-Hasselbalch equation relates the pH of a solution to the pK and concentration of an acid to its conjugate base. Buffered solutions resist changes in pH within about one pH unit of the pK of the buffering ...
chemistry 110 lecture
... PROBLEMS: 1. Calcium hydroxide is reacted with nitric acid. a. How many moles of calcium nitrate is produced when 3 moles of calcium hydroxide and 4 moles of nitric acid are mixed? METHOD: Find the L.R. J Calculate the moles of product that can be produced from each reactant ...
... PROBLEMS: 1. Calcium hydroxide is reacted with nitric acid. a. How many moles of calcium nitrate is produced when 3 moles of calcium hydroxide and 4 moles of nitric acid are mixed? METHOD: Find the L.R. J Calculate the moles of product that can be produced from each reactant ...
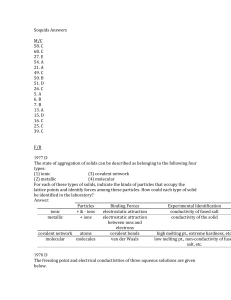
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... CaCO3(s) + H+(aq) Ca2+(aq) + CO2(g) + H2O(l) (b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pre ...
... CaCO3(s) + H+(aq) Ca2+(aq) + CO2(g) + H2O(l) (b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pre ...
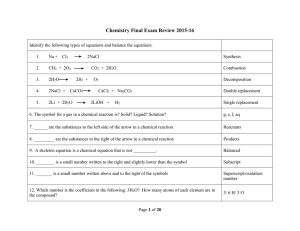
Type Of Chemical Reaction
... 90. _____ are substances that may change color in the presence of an acid or base ...
... 90. _____ are substances that may change color in the presence of an acid or base ...
Honors Chemistry Chapter 14 notes—Acids, Bases, and pH I. Acids
... the base and the ions like in the acid. 3. Bases that accept H+ a. Bronsted-Lowery definition of a base—a substance that accepts H+ b. Best example is probably ammonia, NH3 1) Ammonia gas dissolves in water and reacts with the water and then dissociates 2) NH3(g) + H2O(l) NH4+(aq) + OH-(aq) 4. Ba ...
... the base and the ions like in the acid. 3. Bases that accept H+ a. Bronsted-Lowery definition of a base—a substance that accepts H+ b. Best example is probably ammonia, NH3 1) Ammonia gas dissolves in water and reacts with the water and then dissociates 2) NH3(g) + H2O(l) NH4+(aq) + OH-(aq) 4. Ba ...
Acids and bases
... energy (negative sign) Endothermic reactions absorb heat so they have energy added (positive sign) ...
... energy (negative sign) Endothermic reactions absorb heat so they have energy added (positive sign) ...
Slide 1 - Mrs. Reed Science Classes
... percentage yield of magnesium chloride if 100. g of magnesium react with excess hydrochloric acid to yield 330. g of magnesium chloride. a. 71.8% c. 81.6% b. 74.3% d. 84.2% ...
... percentage yield of magnesium chloride if 100. g of magnesium react with excess hydrochloric acid to yield 330. g of magnesium chloride. a. 71.8% c. 81.6% b. 74.3% d. 84.2% ...
File
... Hydrogen gas and bromine liquid reaction to form gaseous hydrogen bromide. The equilibrium constant for the reaction is 4 x 1018. 1. Calculate the free energy change at 200°C 2. Calculate the free energy change if the partial pressure of HBr is 1.50 atm and hydrogen is 0.500 atm at 298 K. The standa ...
... Hydrogen gas and bromine liquid reaction to form gaseous hydrogen bromide. The equilibrium constant for the reaction is 4 x 1018. 1. Calculate the free energy change at 200°C 2. Calculate the free energy change if the partial pressure of HBr is 1.50 atm and hydrogen is 0.500 atm at 298 K. The standa ...
Ch 11 Chemical Reactions
... Nitric acid dissolved in water reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate dissolved in water. ...
... Nitric acid dissolved in water reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate dissolved in water. ...
200 ways to pass the regents
... 151. The metals above H2 on Table J will react with acids to make H2 gas bubbles. 152. Arrhenius says: “Acids give off H+ or H3O+ ions in solution.” “Bases give off OH- ions in solution.” 153. Brønsted says: “Acids donate protons.” “Bases accept protons.” 154. Acids and bases react in neutralization ...
... 151. The metals above H2 on Table J will react with acids to make H2 gas bubbles. 152. Arrhenius says: “Acids give off H+ or H3O+ ions in solution.” “Bases give off OH- ions in solution.” 153. Brønsted says: “Acids donate protons.” “Bases accept protons.” 154. Acids and bases react in neutralization ...
rain drops as an alternative electrical energy source
... The flow of rainwater in the drainpipe has a certain speed. We could make assumption that the roof height is H. In the H position the water has potential energy and kinetic energy. After a lot of rainwater collection from the roof, the water will drop through the drainpipe. The paddles are placed un ...
... The flow of rainwater in the drainpipe has a certain speed. We could make assumption that the roof height is H. In the H position the water has potential energy and kinetic energy. After a lot of rainwater collection from the roof, the water will drop through the drainpipe. The paddles are placed un ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.