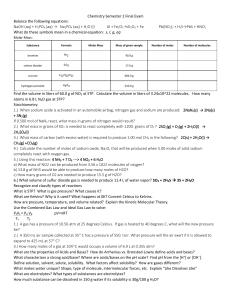
Practice Test 2
... The correct complete ionic equation for the reaction that occurs when aqueous solutions of Ca(NO3)2 and Na2CO3 are mixed is A) Ca(NO3)2(aq) + Na2CO3(aq) ----> CaCO3(s) + 2 NaNO3(aq) B) Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + CO32-(aq) ----> CaCO3(s) + 2 Na+(aq) + 2 NO3-(aq) C) Ca2+(aq) + 2 NO3-(aq) + 2 ...
... The correct complete ionic equation for the reaction that occurs when aqueous solutions of Ca(NO3)2 and Na2CO3 are mixed is A) Ca(NO3)2(aq) + Na2CO3(aq) ----> CaCO3(s) + 2 NaNO3(aq) B) Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + CO32-(aq) ----> CaCO3(s) + 2 Na+(aq) + 2 NO3-(aq) C) Ca2+(aq) + 2 NO3-(aq) + 2 ...
111 Exam I F 04 use
... Tear off this top page (pg. 1)-It is your scratch paper The following are molar masses you may or may not need: H2O = 18.02 ...
... Tear off this top page (pg. 1)-It is your scratch paper The following are molar masses you may or may not need: H2O = 18.02 ...
File
... D. Venting some CO2 gas from the flask 111. In a sealed bottle that is half full of water, equilibrium will be attained when water molecules A. Cease to evaporate B. Begin to condense C. Are equal in number for both the liquid and the gas phase D. Evaporate and condense at equal rates 112. At equili ...
... D. Venting some CO2 gas from the flask 111. In a sealed bottle that is half full of water, equilibrium will be attained when water molecules A. Cease to evaporate B. Begin to condense C. Are equal in number for both the liquid and the gas phase D. Evaporate and condense at equal rates 112. At equili ...
handout 4
... Lecture Example: Carbon disulfide (CS2) burns in oxygen according to the following equation. CS2 + 3 O2 → CO2 + 2 SO2 Calculate the moles of SO2 each component present in the flask at the end of the reaction when 3.0 mol of CS2 and 3.0 mol of O2 are mixed. ...
... Lecture Example: Carbon disulfide (CS2) burns in oxygen according to the following equation. CS2 + 3 O2 → CO2 + 2 SO2 Calculate the moles of SO2 each component present in the flask at the end of the reaction when 3.0 mol of CS2 and 3.0 mol of O2 are mixed. ...
Chemistry at Karlsruhe 1860
... • Prof. Justus Liebig and his group developed methods to analyze organic chemical • Like the Berzelians they dealt only with empirical data and equivalent weights • Organic Compounds generally are made up of carbon, hydrogen and oxygen • Organic compounds when combusted in air form water, and carbon ...
... • Prof. Justus Liebig and his group developed methods to analyze organic chemical • Like the Berzelians they dealt only with empirical data and equivalent weights • Organic Compounds generally are made up of carbon, hydrogen and oxygen • Organic compounds when combusted in air form water, and carbon ...
MULTIPLE CHOICE
... A) Cu (s) + 2AgNO3 (aq) 2Ag (s) + Cu(NO3 )2 (aq) B) HCl (aq) + NaOH (aq) → H 2 O (l) + NaCl (aq) C) AgNO3 (aq) + HCl (aq) AgCl (s) + HNO3 (aq) D) Ba(C2 H3O2 )2 (aq) + Na 2SO4 (aq) BaSO4 (s) + 2NaC2 H3O2 (aq) E) H2 CO3 (aq) + Ca(NO3 )2 (aq) 2HNO3 (aq) + CaCO3 (s) 49) Which one of the followin ...
... A) Cu (s) + 2AgNO3 (aq) 2Ag (s) + Cu(NO3 )2 (aq) B) HCl (aq) + NaOH (aq) → H 2 O (l) + NaCl (aq) C) AgNO3 (aq) + HCl (aq) AgCl (s) + HNO3 (aq) D) Ba(C2 H3O2 )2 (aq) + Na 2SO4 (aq) BaSO4 (s) + 2NaC2 H3O2 (aq) E) H2 CO3 (aq) + Ca(NO3 )2 (aq) 2HNO3 (aq) + CaCO3 (s) 49) Which one of the followin ...
Chemical Reactions
... we were to decrease pressure by increasing volume, the equilibrium of the above reaction will shift to the left, because the reactant side has greater number of moles than does the product side. The system tries to counteract the decrease in partial pressure of gas molecules by shifting to the side ...
... we were to decrease pressure by increasing volume, the equilibrium of the above reaction will shift to the left, because the reactant side has greater number of moles than does the product side. The system tries to counteract the decrease in partial pressure of gas molecules by shifting to the side ...
Experiment 11 CHEMICAL REACTIONS
... of your spot plates as indicated below and look for evidence of a chemical reaction. (If a precipitate is formed, be sure to write the color of the precipitate.) Record your observations in Table 11.1. (If no reaction occurs, write N.R. in the table.) Save the reaction mixtures until after you have ...
... of your spot plates as indicated below and look for evidence of a chemical reaction. (If a precipitate is formed, be sure to write the color of the precipitate.) Record your observations in Table 11.1. (If no reaction occurs, write N.R. in the table.) Save the reaction mixtures until after you have ...
Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
... 1.) When sodium azide is activated in an automobile airbag, nitrogen gas and sodium are produced: 2NaN3(s) → 2Na(s) + 3N2(g) If 0.500 mol of NaN3 react, what mass in grams of nitrogen would result? 21.0 g 2.) What mass in grams of SO2 is needed to react completely with 1200. grams of O2 ? 2SO2(g) + ...
... 1.) When sodium azide is activated in an automobile airbag, nitrogen gas and sodium are produced: 2NaN3(s) → 2Na(s) + 3N2(g) If 0.500 mol of NaN3 react, what mass in grams of nitrogen would result? 21.0 g 2.) What mass in grams of SO2 is needed to react completely with 1200. grams of O2 ? 2SO2(g) + ...
IGCSE SoW 2013
... Describe the addition reaction of alkenes with bromine, including the decolourising of bromine water as a test for alkenes ...
... Describe the addition reaction of alkenes with bromine, including the decolourising of bromine water as a test for alkenes ...
Chemical Reactions
... In the case of water, many times only one H will be replaced leaving a hydroxide ion combined with the replacing element ...
... In the case of water, many times only one H will be replaced leaving a hydroxide ion combined with the replacing element ...
Chapters 13 and 14
... b. When table salt (NaCl) and sugar (C12H22O11) are dissolved in water, it is observed that i. Both solutions have a higher boiling point than pure water, and ii. The boiling point of 0.10 M NaCl (aq) is higher than that of 0.10 M C12H22O11 (aq). c. Methane gas does not behave as an ideal gas at low ...
... b. When table salt (NaCl) and sugar (C12H22O11) are dissolved in water, it is observed that i. Both solutions have a higher boiling point than pure water, and ii. The boiling point of 0.10 M NaCl (aq) is higher than that of 0.10 M C12H22O11 (aq). c. Methane gas does not behave as an ideal gas at low ...
H - JMap
... 40 When NH4NO3 is added to water, an acidic solution forms. This process is referred to as (1) dehydration (3) hydrolysis (2) electrolysis (4) neutralization 41 Which solution is the best conductor of electricity? (1) 0.1 M HCl(aq) (2) 0.1 M CH3OH(aq) (3) 0.1 M NH3(aq) (4) 0.1 M CH3COOH(aq) ...
... 40 When NH4NO3 is added to water, an acidic solution forms. This process is referred to as (1) dehydration (3) hydrolysis (2) electrolysis (4) neutralization 41 Which solution is the best conductor of electricity? (1) 0.1 M HCl(aq) (2) 0.1 M CH3OH(aq) (3) 0.1 M NH3(aq) (4) 0.1 M CH3COOH(aq) ...
Water
... They will come together and are held by Hydrophobic bonds. There are formed because of the size of proteins molecules because they want to avoid water (fear water). They leave water part and come together. ...
... They will come together and are held by Hydrophobic bonds. There are formed because of the size of proteins molecules because they want to avoid water (fear water). They leave water part and come together. ...
Two moles of gas at 1 bar and 298 K are compressed at constant T
... DEFINITION of Standard Heat of Formation of Compounds: This is the heat required to form the compound [in its most stable form] from its constituent elements in their standard states. Thus we need to know the most stable state of the compound and elements in order to be able to write the formation ...
... DEFINITION of Standard Heat of Formation of Compounds: This is the heat required to form the compound [in its most stable form] from its constituent elements in their standard states. Thus we need to know the most stable state of the compound and elements in order to be able to write the formation ...
Final Review 2006
... ____ 30. Which observation does NOT indicate that a chemical reaction has occurred? a. formation of a precipitate c. evolution of heat and light b. production of a gas d. change in total mass of substances ____ 31. A solid produced by a chemical reaction in solution that separates from the solution ...
... ____ 30. Which observation does NOT indicate that a chemical reaction has occurred? a. formation of a precipitate c. evolution of heat and light b. production of a gas d. change in total mass of substances ____ 31. A solid produced by a chemical reaction in solution that separates from the solution ...
Notes on Chapter 12 Chemical Equilibrium
... c. energy-the collision must provide sufficient energy to supply the energy of activation for the chemical reaction (i.e. the energy required to break and form new bonds). ...
... c. energy-the collision must provide sufficient energy to supply the energy of activation for the chemical reaction (i.e. the energy required to break and form new bonds). ...
Chapters 14 and 15 Outline
... Amphoteric – any species that can react as either an Neutralization – is the reaction between ...
... Amphoteric – any species that can react as either an Neutralization – is the reaction between ...
Red-ox reactions Electochemistry
... element that is oxidised. Show the increase in oxidation number per atom. Draw a bracket to connect atoms of the element that is reduced. Show the decrease in oxidation number per atom. b) determined the factors that will make the total increase and decrease in oxidation numbers ...
... element that is oxidised. Show the increase in oxidation number per atom. Draw a bracket to connect atoms of the element that is reduced. Show the decrease in oxidation number per atom. b) determined the factors that will make the total increase and decrease in oxidation numbers ...
Sections 6.4 - 6.5
... • Aluminum: use in the automotive and aerospace industry as DURALUMINIUM alloyed with Mg and Cu; in ship building as HYDRONALIUM, alloyed with 3-12 % Mg – with disastrous consequences in the BC SeaCat Ferry building program and the Falkland War: Al/Mg + n O2(g) → Al2O3 + MgO + lots of heat ! in wate ...
... • Aluminum: use in the automotive and aerospace industry as DURALUMINIUM alloyed with Mg and Cu; in ship building as HYDRONALIUM, alloyed with 3-12 % Mg – with disastrous consequences in the BC SeaCat Ferry building program and the Falkland War: Al/Mg + n O2(g) → Al2O3 + MgO + lots of heat ! in wate ...
Camp 1 - Quynh Nguyen Official Website
... Separation of the parts of a mixture by heating a liquid solution until one component boils, changing into the gaseous state. The pure substance in the gaseous state is then collected and cooled into the liquid state. Boiling is a physical change. Distillation allows components in a homogeneous mixt ...
... Separation of the parts of a mixture by heating a liquid solution until one component boils, changing into the gaseous state. The pure substance in the gaseous state is then collected and cooled into the liquid state. Boiling is a physical change. Distillation allows components in a homogeneous mixt ...
Specification
... Amount of Substance This is a physical quantity, symbol n (italic n), measured in a unit called the mole, which has the abbreviation mol. The term, ‘number of moles’ is to be avoided. The term, ‘amount of substance in moles’ is preferred. In the same manner, the size of an object can be described in ...
... Amount of Substance This is a physical quantity, symbol n (italic n), measured in a unit called the mole, which has the abbreviation mol. The term, ‘number of moles’ is to be avoided. The term, ‘amount of substance in moles’ is preferred. In the same manner, the size of an object can be described in ...
WELCOME TO AP CHEMISTRY
... 54. A sample of dolomitic limestone containing only CaCO3 and MgCO3 was analyzed. (a) When a 0.2800 gram sample of this limestone was decomposed by heating, 0.00308 moles of CO2 were evolved. How many grams of CO2 were produced? ...
... 54. A sample of dolomitic limestone containing only CaCO3 and MgCO3 was analyzed. (a) When a 0.2800 gram sample of this limestone was decomposed by heating, 0.00308 moles of CO2 were evolved. How many grams of CO2 were produced? ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.