2011-2012 ACAD REVIEW SHEET Chapter 16
... homogeneous equilibria heterogeneous equilibria reaction quotient Le Chatelier’s principle Haber process ...
... homogeneous equilibria heterogeneous equilibria reaction quotient Le Chatelier’s principle Haber process ...
Chemistry Merit Badge
... A) Visit a laboratory and talk to a practicing chemist. Ask what the chemist does, and what training and education are needed to work as a chemist. B) Using resources found at the library and in periodicals, books, and the Internet (with your parent’s permission), learn about two different kinds of ...
... A) Visit a laboratory and talk to a practicing chemist. Ask what the chemist does, and what training and education are needed to work as a chemist. B) Using resources found at the library and in periodicals, books, and the Internet (with your parent’s permission), learn about two different kinds of ...
1 Intro / Review : Chemical Kinetics
... Also, the orientations of the reactant molecules during the collision must allow for the rearrangement of reactant bonds to form product bonds. Essential knowledge 4.B.3: A successful collision can be viewed as following a reaction path with an associated energy profile. Enduring understanding 4.D: ...
... Also, the orientations of the reactant molecules during the collision must allow for the rearrangement of reactant bonds to form product bonds. Essential knowledge 4.B.3: A successful collision can be viewed as following a reaction path with an associated energy profile. Enduring understanding 4.D: ...
Organic Chemistry
... carbonyl group. The loading of the (diacetoxyiodo)phenyl group on polystyrene was determined by iodometric titration and calculated to be 1.39 mmol/g. The reaction conditions were optimized for solvent, time and equivalent of PS-DIB using 4-chlorothiophenol as substrate and the yields were determine ...
... carbonyl group. The loading of the (diacetoxyiodo)phenyl group on polystyrene was determined by iodometric titration and calculated to be 1.39 mmol/g. The reaction conditions were optimized for solvent, time and equivalent of PS-DIB using 4-chlorothiophenol as substrate and the yields were determine ...
Synthesis gas purification.
... Due to the large feedstocks variety that can be processed, significant variations of the composition of the synthesis gas are expected. Especially, this affects the nature of the impurities that are present (element, speciation), as well as their relative contents. Moreover, due to high FT catalyst ...
... Due to the large feedstocks variety that can be processed, significant variations of the composition of the synthesis gas are expected. Especially, this affects the nature of the impurities that are present (element, speciation), as well as their relative contents. Moreover, due to high FT catalyst ...
Assistant Professor Chemistry, Class-2, Advt No. 84/2016
... (B) the atomic orbitals should overlap to a considerable extent (C) the symmetry of the combining orbitals should be the same (D) the energy of resulting antibonding orbital is less than that of bonding orbital ...
... (B) the atomic orbitals should overlap to a considerable extent (C) the symmetry of the combining orbitals should be the same (D) the energy of resulting antibonding orbital is less than that of bonding orbital ...
No Slide Title
... Carrying out the first process followed by the second process means that our final state is the same as our initial state. There is no change in the system, and so Htotal = 0.0 kJ/mol. Therefore, the change in enthalpy for the second process must be the same as the change in enthalpy for the first ...
... Carrying out the first process followed by the second process means that our final state is the same as our initial state. There is no change in the system, and so Htotal = 0.0 kJ/mol. Therefore, the change in enthalpy for the second process must be the same as the change in enthalpy for the first ...
Word - Chemistry and More
... b) ammonium sulfide and strontium nitrate c) lithium carbonate and cobalt(III) chloride d) potassium phosphate and sodium hydroxide 10. (Chapter 8) For each case where there was a net reaction in Question 6, write both a balanced molecular equation and a net ionic equation for the reaction. 11. (Cha ...
... b) ammonium sulfide and strontium nitrate c) lithium carbonate and cobalt(III) chloride d) potassium phosphate and sodium hydroxide 10. (Chapter 8) For each case where there was a net reaction in Question 6, write both a balanced molecular equation and a net ionic equation for the reaction. 11. (Cha ...
9 free IB Chem labs (sent to OCC) - VicPark-IBRoundtable-2009
... 4. Obtain a sample of acid (about 10mL) and place it in an Erlenmeyer flask. How are you going to collect this sample to as many significant figures as possible? 5. Obtain at least 8cm of Mg ribbon. 6. When you are ready with the stopwatch and the tubing, place the Mg inside the flask. Immediately s ...
... 4. Obtain a sample of acid (about 10mL) and place it in an Erlenmeyer flask. How are you going to collect this sample to as many significant figures as possible? 5. Obtain at least 8cm of Mg ribbon. 6. When you are ready with the stopwatch and the tubing, place the Mg inside the flask. Immediately s ...
Test 8 Review
... Ideal Gases. In order to study gases, chemists have devised a model. The model is called an ideal gas (a gas which explains the behavior of all gases). This Ideal Gas Kinetic theory of gases (under ideal circumstances) model is based on the assumptions to the right, and can be 1. Gas are composed of ...
... Ideal Gases. In order to study gases, chemists have devised a model. The model is called an ideal gas (a gas which explains the behavior of all gases). This Ideal Gas Kinetic theory of gases (under ideal circumstances) model is based on the assumptions to the right, and can be 1. Gas are composed of ...
H 2 O
... • The distribution of energy deposits vary: the probably of short tracks increases with LET depending on the placement of the clusters of radicals (spurs). • The recombinations in the heterogenous zones are much more probable as the LET is elevated because the concentration of free radicals is very ...
... • The distribution of energy deposits vary: the probably of short tracks increases with LET depending on the placement of the clusters of radicals (spurs). • The recombinations in the heterogenous zones are much more probable as the LET is elevated because the concentration of free radicals is very ...
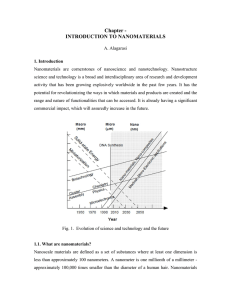
Chapter - INTRODUCTION TO NANOMATERIALS
... functional materials, both inorganic and organic, allowing to manipulate mechanical, catalytic, electric, magnetic, optical and electronic functions. The production of nanophase or cluster-assembled materials is usually based upon the creation of separated small clusters which then are fused into a ...
... functional materials, both inorganic and organic, allowing to manipulate mechanical, catalytic, electric, magnetic, optical and electronic functions. The production of nanophase or cluster-assembled materials is usually based upon the creation of separated small clusters which then are fused into a ...
Worksheet Key
... g) H2 (g) + Cl2 (g) 2 HCl (g): volume is doubled. No change; changing volume or pressure will not affect this system; same # moles on both sides. h) Using the same system as above, some neon is added to the system. No change; neon is an inert gas; it won’t react with or affect the system. ...
... g) H2 (g) + Cl2 (g) 2 HCl (g): volume is doubled. No change; changing volume or pressure will not affect this system; same # moles on both sides. h) Using the same system as above, some neon is added to the system. No change; neon is an inert gas; it won’t react with or affect the system. ...
AP Chemistry - cloudfront.net
... (b) Which point corresponds to the critical point? Which point corresponds to the triple point? (c) What curve corresponds to the conditions at which the solid and gas are in equilibrium? (d) Describe what happens when you start at point H and decrease the pressure at constant temperature. (f) is li ...
... (b) Which point corresponds to the critical point? Which point corresponds to the triple point? (c) What curve corresponds to the conditions at which the solid and gas are in equilibrium? (d) Describe what happens when you start at point H and decrease the pressure at constant temperature. (f) is li ...
It`s Easy Being a Green Chemist
... and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure. 7) Use of Renewable Feedstocks A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable. 8) Reduce Derivatives Unnecessary d ...
... and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure. 7) Use of Renewable Feedstocks A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable. 8) Reduce Derivatives Unnecessary d ...
AQA Additional Sci C2 Revision Guide
... outer shell of its atoms. Elements in groups 1 and 2 of the periodic table only have 1 or 2 electrons in their outer shells so these form positive ions by losing their outer electrons. Elements in groups 6 and 7 of the periodic table only need 1 or 2 electrons to fill up their outer shells so these ...
... outer shell of its atoms. Elements in groups 1 and 2 of the periodic table only have 1 or 2 electrons in their outer shells so these form positive ions by losing their outer electrons. Elements in groups 6 and 7 of the periodic table only need 1 or 2 electrons to fill up their outer shells so these ...
Equilibrium
... contains more reactant than product. In principle, almost all reactions are reversible to some extent under the right conditions. In practice, one set of components is often so favored that the other set cannot be detected. If one set of components (reactants) is completely converted to new substanc ...
... contains more reactant than product. In principle, almost all reactions are reversible to some extent under the right conditions. In practice, one set of components is often so favored that the other set cannot be detected. If one set of components (reactants) is completely converted to new substanc ...
Homework - PHA Science
... III. Review writing and balancing equations, mole and mass stoichiometry IV. Practice Problems Homework: 112 #60c, e, g, h, k and l, 64, ...
... III. Review writing and balancing equations, mole and mass stoichiometry IV. Practice Problems Homework: 112 #60c, e, g, h, k and l, 64, ...
Unit 6: Reactions and Stoichiometry
... Stoichiometry! 1. If 156.0 grams of potassium metal reacts with excess water, then how many grams of potassium hydroxide are formed? What volume of hydrogen gas, in liters, is formed at STP ? __2__K (s) ...
... Stoichiometry! 1. If 156.0 grams of potassium metal reacts with excess water, then how many grams of potassium hydroxide are formed? What volume of hydrogen gas, in liters, is formed at STP ? __2__K (s) ...
2/22 Lecture Slides
... ΔG = Change in Gibbs free energy This tells us if a process is spontaneous (expected to happen) or non-spontaneous ΔG < 0 process is spontaneous (favored) ΔG = ΔH - TΔS (T is absolute temperature) processes that are exothermic (Δ H < 0) and increase disorder (Δ S > 0) are favored at all T processes ...
... ΔG = Change in Gibbs free energy This tells us if a process is spontaneous (expected to happen) or non-spontaneous ΔG < 0 process is spontaneous (favored) ΔG = ΔH - TΔS (T is absolute temperature) processes that are exothermic (Δ H < 0) and increase disorder (Δ S > 0) are favored at all T processes ...
Document
... Just be aware that when we write balanced equations on paper, we don’t put 1 coefficients (by writing a formula down, we are stating that there is one of those molecules present ). ...
... Just be aware that when we write balanced equations on paper, we don’t put 1 coefficients (by writing a formula down, we are stating that there is one of those molecules present ). ...