Chemistry - Plymouth Public Schools
... MA CHM 4.6 Name and write the chemical formulas for simple ionic and molecular compounds, including those that contain the polyatomic ions: ammonium, carbonate, hydroxide, nitrate, phosphate, and sulfate. Chemical Reactions and Stoichiometry Central Concepts: In a chemical reaction, one or more reac ...
... MA CHM 4.6 Name and write the chemical formulas for simple ionic and molecular compounds, including those that contain the polyatomic ions: ammonium, carbonate, hydroxide, nitrate, phosphate, and sulfate. Chemical Reactions and Stoichiometry Central Concepts: In a chemical reaction, one or more reac ...
Sec 5.8 - 5.11 notes
... 11) The salt bridge can contain any _ELECTROLYTES________. 12) The anode will __LOSE_____(gains/loses) mass as it is _OXIDIZED___(oxidized/reduced). 13) The cathode will _GAIN____ mass as it is __REDUCED_____(oxidized/reduced). ...
... 11) The salt bridge can contain any _ELECTROLYTES________. 12) The anode will __LOSE_____(gains/loses) mass as it is _OXIDIZED___(oxidized/reduced). 13) The cathode will _GAIN____ mass as it is __REDUCED_____(oxidized/reduced). ...
B.Sc. (Hons.) CHEMISTRY THREE-YEARS FULL
... of combustion and its applications; calculation of bond energy, bond dissociation energy and resonance energy from thermochemical data, effect of temperature (Kirchhoff’s equations) and pressure on enthalpy of reactions. Adiabatic flame temperature, explosion temperature. Second Law: Concept of entr ...
... of combustion and its applications; calculation of bond energy, bond dissociation energy and resonance energy from thermochemical data, effect of temperature (Kirchhoff’s equations) and pressure on enthalpy of reactions. Adiabatic flame temperature, explosion temperature. Second Law: Concept of entr ...
Sustainable Oxidation Catalysis for Synthesis
... At present, many oxidation reactions rely on inefficient and wasteful methods that are problematic on a larger scale. There is a need to develop efficient catalysts that use sustainable terminal oxidants such as molecular oxygen or hydrogen peroxide. Although such methods are employed in the prepara ...
... At present, many oxidation reactions rely on inefficient and wasteful methods that are problematic on a larger scale. There is a need to develop efficient catalysts that use sustainable terminal oxidants such as molecular oxygen or hydrogen peroxide. Although such methods are employed in the prepara ...
Chemical Equations and Reactions
... A coefficient multiplies the number of atoms of each element indicated in a chemical formula. Thus, 2H2O represents four H atoms and two O atoms. To add two more hydrogen atoms to the right side of the equation, one may be tempted to change the subscript in the formula of water so that H2O becomes H ...
... A coefficient multiplies the number of atoms of each element indicated in a chemical formula. Thus, 2H2O represents four H atoms and two O atoms. To add two more hydrogen atoms to the right side of the equation, one may be tempted to change the subscript in the formula of water so that H2O becomes H ...
Material Equilibrium
... ENTROPY AND EQUILIBRIUM Example: In isolated system (not in material equilibrium) The spontaneous chemical reaction or transport of matter are irreversible process that increase the ENTROPY The process was continued until the system’s entropy is maximized. Once it is maximized, any further pr ...
... ENTROPY AND EQUILIBRIUM Example: In isolated system (not in material equilibrium) The spontaneous chemical reaction or transport of matter are irreversible process that increase the ENTROPY The process was continued until the system’s entropy is maximized. Once it is maximized, any further pr ...
AP Chemistry
... c. When comparing two products (on the right side of the chart), the product that is listed (higher/lower) on the chart is the stronger reducing agent. d. The strongest reducing agents are found in column (1/17) on the periodic table. e. The strongest oxidizing agents are found in column (1/17) on t ...
... c. When comparing two products (on the right side of the chart), the product that is listed (higher/lower) on the chart is the stronger reducing agent. d. The strongest reducing agents are found in column (1/17) on the periodic table. e. The strongest oxidizing agents are found in column (1/17) on t ...
Chemistry notes Important terms *Mass of element in a sample
... Factors that influence reaction rate 1. Concentration – molecules must collide to react a. Reaction rate is proportional to concentration of reaction 2. physical state – molecules must mix to collide a. the more finely divided a solid or liquid reaction , the greater its surface area per unit volume ...
... Factors that influence reaction rate 1. Concentration – molecules must collide to react a. Reaction rate is proportional to concentration of reaction 2. physical state – molecules must mix to collide a. the more finely divided a solid or liquid reaction , the greater its surface area per unit volume ...
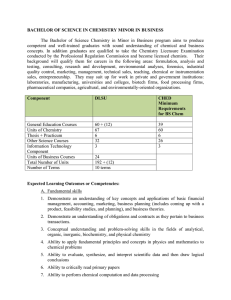
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... competent and well-trained graduates with sound understanding of chemical and business concepts. In addition graduates are qualified to take the Chemistry Licensure Examination conducted by the Professional Regulation Commission and become licensed chemists. Their background will qualify them for ca ...
... competent and well-trained graduates with sound understanding of chemical and business concepts. In addition graduates are qualified to take the Chemistry Licensure Examination conducted by the Professional Regulation Commission and become licensed chemists. Their background will qualify them for ca ...
Chapter 19 Chemical Thermodynamics
... Analyze: We are given four equations and asked to predict the sign of ΔS for each chemical reaction. Plan: The sign of ΔS will be positive if there is an increase in temperature, an increase in the volume in which the molecules move, or an increase in the number of gas particles in the reaction. The ...
... Analyze: We are given four equations and asked to predict the sign of ΔS for each chemical reaction. Plan: The sign of ΔS will be positive if there is an increase in temperature, an increase in the volume in which the molecules move, or an increase in the number of gas particles in the reaction. The ...
Last Name Professor BEAMER First Name
... Step 2: Use mole ratios to convert moles KNOWN moles UNK ...
... Step 2: Use mole ratios to convert moles KNOWN moles UNK ...
Problem 1: A brief history of life in the universe
... consisting of over 100 billion stars, is rich in hydrogen. The distance between stars is several light years on the average. Hydrogen is also the major constituent of the interstellar space. There are about 100 billion galaxies in the universe. The empty space between galaxies is vast. For example, ...
... consisting of over 100 billion stars, is rich in hydrogen. The distance between stars is several light years on the average. Hydrogen is also the major constituent of the interstellar space. There are about 100 billion galaxies in the universe. The empty space between galaxies is vast. For example, ...
Advanced Higher Chemistry Resource Guide
... This resource guide has been produced in response to requests from staff who attended the NQ Sciences events at Hampden Stadium in December 2013. Those attending felt it would be useful to have a document which helped them navigate to the most relevant resources quickly. The following pages show the ...
... This resource guide has been produced in response to requests from staff who attended the NQ Sciences events at Hampden Stadium in December 2013. Those attending felt it would be useful to have a document which helped them navigate to the most relevant resources quickly. The following pages show the ...
C:\Documents and Settings\mrh70950\My Documents
... 1. Structural formula hybrids of types A and B are called partially condensed formulas. D. Carbon skeleton formulas (also called bond-line formulas) 1. Every line is a covalent bond 2. Every intersection of two or more lines is a C 3. Every terminus of a line is a C unless another atom explicitly re ...
... 1. Structural formula hybrids of types A and B are called partially condensed formulas. D. Carbon skeleton formulas (also called bond-line formulas) 1. Every line is a covalent bond 2. Every intersection of two or more lines is a C 3. Every terminus of a line is a C unless another atom explicitly re ...
3 ON THE THERMODYNAMICS OF FATTY ACID OXIDATION
... Abstract. The energy yield from the combustion of fatty acids and the ATP yield of their oxidation increases with the length of the acyl chain and decreases with unsaturation. Simple expressions describing these relationships are compared with data from the literature, and expressions for the yield ...
... Abstract. The energy yield from the combustion of fatty acids and the ATP yield of their oxidation increases with the length of the acyl chain and decreases with unsaturation. Simple expressions describing these relationships are compared with data from the literature, and expressions for the yield ...
Biodiesel Production and Fuel Quality_JVG
... number and density, reflect the properties of the chemical compounds that make up biodiesel, other properties provide indications of the quality of the production process. Generally, the parameters given in ASTM D6751 are defined by other ASTM standards. However, other test methods, such as those d ...
... number and density, reflect the properties of the chemical compounds that make up biodiesel, other properties provide indications of the quality of the production process. Generally, the parameters given in ASTM D6751 are defined by other ASTM standards. However, other test methods, such as those d ...
Document
... Chapters are helpfully signposted throughout, informing the reader how topics are related, which is especially important in such a multidisciplinary subject. Topics are also presented clearly and with a logical progression culminating in the main points, questions and reading sections at the beginni ...
... Chapters are helpfully signposted throughout, informing the reader how topics are related, which is especially important in such a multidisciplinary subject. Topics are also presented clearly and with a logical progression culminating in the main points, questions and reading sections at the beginni ...
Ethics of Chemical Synthesis - HYLE-
... group or community leader or a court, depending on its social structure. In addition, the institution may be internally represented by one’s own conscience, which is even required if the institution is not formaly established as it is the case with humanity.5 Thus, we may once more distinguish betwe ...
... group or community leader or a court, depending on its social structure. In addition, the institution may be internally represented by one’s own conscience, which is even required if the institution is not formaly established as it is the case with humanity.5 Thus, we may once more distinguish betwe ...
Topic 14 - Fertilisers
... Ammonia has a characteristic pungent smell. Ammonia is very soluble in water as shown by the fountain experiment. ...
... Ammonia has a characteristic pungent smell. Ammonia is very soluble in water as shown by the fountain experiment. ...