C2 Chemistry - Burton Borough School
... ATOMIC NUMBER (proton number/the small one) The number of outer shell electrons match the group the element is found in. E.g. Lithium 2,1 is a group 1 element. ...
... ATOMIC NUMBER (proton number/the small one) The number of outer shell electrons match the group the element is found in. E.g. Lithium 2,1 is a group 1 element. ...
Chemical Equations
... • Coefficients tell us the proportions that the molecules need to react in. • They show how matter is conserved ...
... • Coefficients tell us the proportions that the molecules need to react in. • They show how matter is conserved ...
Ch. 3 - Chemical Reactions
... two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas. ...
... two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas. ...
Chapter 1
... D. The Reason Equations Must be Balanced *Notes-The Law of Conservation of Mass dictates that chemical equations must be balanced because atoms are never _____lost__or _____gained__in a chemical reaction. ...
... D. The Reason Equations Must be Balanced *Notes-The Law of Conservation of Mass dictates that chemical equations must be balanced because atoms are never _____lost__or _____gained__in a chemical reaction. ...
Chapter 6-student notes
... 3. A solid has a mass of 35g. When it is mixed with a solution, a chemical reaction happens. If the total mass of the products is 85g, what was the mass of the solution? 4. Solution A gas a mass of 60g. Solution B has a mass of 40g. When they are mixed, a chemical reaction occurs in which a gas is p ...
... 3. A solid has a mass of 35g. When it is mixed with a solution, a chemical reaction happens. If the total mass of the products is 85g, what was the mass of the solution? 4. Solution A gas a mass of 60g. Solution B has a mass of 40g. When they are mixed, a chemical reaction occurs in which a gas is p ...
File
... product, or between moles of products. In a typical stoichiometric problem, the given quantity (starting quantity) is first converted to moles. Then the mole ratio from the balanced equation is used to calculate the moles of the wanted substance. Finally, the moles are converted to any other unit of ...
... product, or between moles of products. In a typical stoichiometric problem, the given quantity (starting quantity) is first converted to moles. Then the mole ratio from the balanced equation is used to calculate the moles of the wanted substance. Finally, the moles are converted to any other unit of ...
Chapter 2: Chemical Reactions Section 1
... energy; they also come into contact more often; lowering temperature slows things down Concentration – amount of substance in a given volume; increased concentration-increased reaction Catalysts – increases the rate of a reaction by decreasing the energy needed to start – Enzymes: biological catalys ...
... energy; they also come into contact more often; lowering temperature slows things down Concentration – amount of substance in a given volume; increased concentration-increased reaction Catalysts – increases the rate of a reaction by decreasing the energy needed to start – Enzymes: biological catalys ...
aq - FCS Physics and Chemistry
... Will Occur In the 2nd column of Table J is a list of nonmetals A nonmetal will replace a less active nonmetal in a ...
... Will Occur In the 2nd column of Table J is a list of nonmetals A nonmetal will replace a less active nonmetal in a ...
Practical and selective aerobic oxidation of alcohols to
... reactor, staged injection of oxygen was used to maintain a consistent amount of gas along the reaction bed. Although characteristics of the flow system were very well-defined, the generality of the system was not demonstrated, as only benzyl alcohol was examined as the substrate. In this work, a com ...
... reactor, staged injection of oxygen was used to maintain a consistent amount of gas along the reaction bed. Although characteristics of the flow system were very well-defined, the generality of the system was not demonstrated, as only benzyl alcohol was examined as the substrate. In this work, a com ...
Chemistry DCA Review Sheet
... 13. Label the following on the Periodic Table: periods, groups (families), metals, non-metals, metalloids, where protons and protons + neutrons can be found. ...
... 13. Label the following on the Periodic Table: periods, groups (families), metals, non-metals, metalloids, where protons and protons + neutrons can be found. ...
PDF(343KB)
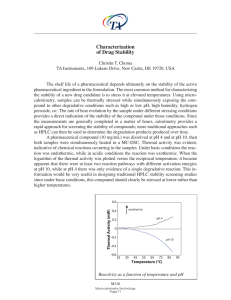
... the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions such as high or low pH, high humidity, hydrogen peroxide, etc. The rate of heat evoluti ...
... the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions such as high or low pH, high humidity, hydrogen peroxide, etc. The rate of heat evoluti ...
Chemical Reactions and Equations
... Starting with elements that only occur in one substance on each side of the equation, make sure that each side of the equation has an equal # of that element. Proceed with all elements. Remember that changing the # of one element may alter elements that have already been ...
... Starting with elements that only occur in one substance on each side of the equation, make sure that each side of the equation has an equal # of that element. Proceed with all elements. Remember that changing the # of one element may alter elements that have already been ...
Chemical Reactions
... There are many kinds of chemical reactions and several ways to classify them. One useful method of classifies reactions into four major types. These are: 1.) synthesis; 2.) decomposition; 3.) single replacement; and 4.) double replacement reactions. Not all reactions can be put into one of these cat ...
... There are many kinds of chemical reactions and several ways to classify them. One useful method of classifies reactions into four major types. These are: 1.) synthesis; 2.) decomposition; 3.) single replacement; and 4.) double replacement reactions. Not all reactions can be put into one of these cat ...
Chemical Reactions
... start are called the reactants and the new substances formed are called the products. Example: When potassium is mixed with water a purple solution of potassium hydroxide is produced and hydrogen gas is given off and catches fire. Reactants: potassium and water ...
... start are called the reactants and the new substances formed are called the products. Example: When potassium is mixed with water a purple solution of potassium hydroxide is produced and hydrogen gas is given off and catches fire. Reactants: potassium and water ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... Furan derivatives can be efficiently converted into other heterocycles. Thus, in 1930th professor of Moscow State University Yu. K. Yuriev developed industrial transformation of furans into pyrroles under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory ...
... Furan derivatives can be efficiently converted into other heterocycles. Thus, in 1930th professor of Moscow State University Yu. K. Yuriev developed industrial transformation of furans into pyrroles under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory ...
CHEMISTRY EXAM 2 REVIEW
... My child completed this review and studied for at least 30 minutes. Define the following chemistry terms: [Chemistry Dictionary] 1. alloy a mixture of metals 2. brittleness the property of matter that is how easily the substance breaks or shatters when force is applied to it. 3. compound a substance ...
... My child completed this review and studied for at least 30 minutes. Define the following chemistry terms: [Chemistry Dictionary] 1. alloy a mixture of metals 2. brittleness the property of matter that is how easily the substance breaks or shatters when force is applied to it. 3. compound a substance ...
Practice with Chemical Equilibrium (Chapter 14) (Due 2/17)
... Practice with Chemical Equilibrium (Chapter 14) (Due 2/17) Here are four additional practice exercises concerning chemical equilibrium (Chapter 14). See your instructor if you have questions. Note that for these questions, the symbol "=" is used to indicate a reversible reaction. Your textbook uses ...
... Practice with Chemical Equilibrium (Chapter 14) (Due 2/17) Here are four additional practice exercises concerning chemical equilibrium (Chapter 14). See your instructor if you have questions. Note that for these questions, the symbol "=" is used to indicate a reversible reaction. Your textbook uses ...
S2-2-07 - Classifying Chemical Reactions
... from notes. Think of other analogies for this type of reaction and fill in notes sheet. Teacher’s analogy: Blackeye + Pinkpanther Blackpanther + Pinkeye. (10 minutes) Have students follow the directions found with material at the center of their table and perform the double displacement reaction B ...
... from notes. Think of other analogies for this type of reaction and fill in notes sheet. Teacher’s analogy: Blackeye + Pinkpanther Blackpanther + Pinkeye. (10 minutes) Have students follow the directions found with material at the center of their table and perform the double displacement reaction B ...