Document
... 25. Justify why or why not we should pursue an energy program of nuclear fusion in the United States. You need to explain the differences in fission and fusion, site advantages and disadvantages of each. Fossil fuels pollute and generate CO2 (responsible for global warming) and are diminishing in a ...
... 25. Justify why or why not we should pursue an energy program of nuclear fusion in the United States. You need to explain the differences in fission and fusion, site advantages and disadvantages of each. Fossil fuels pollute and generate CO2 (responsible for global warming) and are diminishing in a ...
mass number - Knittig Science
... • All living things have some carbon 14 naturally occurring. • When the organism dies, carbon 14 starts to decay • Can look at how much carbon 14 is left and see how long ago the organism lived (using half-life) ...
... • All living things have some carbon 14 naturally occurring. • When the organism dies, carbon 14 starts to decay • Can look at how much carbon 14 is left and see how long ago the organism lived (using half-life) ...
Powerpoint covering atomic structure and isotopes
... ideas about the existence of atoms over 200 years ago. However, it is only relatively recently that special microscopes (called electron microscopes) have been invented that can actually ‘see’ atoms. This image is highly magnified. What could it be showing? The yellow blobs are individual gold atoms ...
... ideas about the existence of atoms over 200 years ago. However, it is only relatively recently that special microscopes (called electron microscopes) have been invented that can actually ‘see’ atoms. This image is highly magnified. What could it be showing? The yellow blobs are individual gold atoms ...
Chapter 4
... d. that in a copper penny, there is one atom for every person on Earth The range in size of most atomic radii is approximately ____. a. 2 to 5 cm c. 5 10 m to 2 10 m b. 2 to 5 nm d. 5 10 m to 2 10 m Dalton hypothesized that atoms are indivisible and that all atoms of an element are identical. It is ...
... d. that in a copper penny, there is one atom for every person on Earth The range in size of most atomic radii is approximately ____. a. 2 to 5 cm c. 5 10 m to 2 10 m b. 2 to 5 nm d. 5 10 m to 2 10 m Dalton hypothesized that atoms are indivisible and that all atoms of an element are identical. It is ...
RLE-TR-078-047086
... last vibration, v3, has a first excited state above the ground state by about 10 kT so that the Boltzmann factor allows only a very small population of this level. For this reason we have not observed any absorption due to the molecule in the first excited v 3 vibrational mode. The observed absorpti ...
... last vibration, v3, has a first excited state above the ground state by about 10 kT so that the Boltzmann factor allows only a very small population of this level. For this reason we have not observed any absorption due to the molecule in the first excited v 3 vibrational mode. The observed absorpti ...
History of the Atom Model
... the smallest fundamental units of matter. All elements consist of atoms which are indivisible & indestructible. All atoms of the same element are identical in size, shape & mass. ...
... the smallest fundamental units of matter. All elements consist of atoms which are indivisible & indestructible. All atoms of the same element are identical in size, shape & mass. ...
University of Groningen Atom Trap Trace Analysis of Calcium
... for the first time in 1992 by Kurosu and Shimizu [73]. Other members of the alkalineearth atom group are beryllium, magnesium, strontium, barium and radium. These atoms share some interesting properties for cold atom research. The spin-forbidden intercombination lines between the singlet and triplet ...
... for the first time in 1992 by Kurosu and Shimizu [73]. Other members of the alkalineearth atom group are beryllium, magnesium, strontium, barium and radium. These atoms share some interesting properties for cold atom research. The spin-forbidden intercombination lines between the singlet and triplet ...
Early Atomic History
... chemical composition of many compounds. He found that a given compound always contains the exact same proportion of elements by mass. This is known as the law of definite proportion. For example, all samples of water contain 88.8% oxygen by mass, and 11.2% hydrogen by mass. ...
... chemical composition of many compounds. He found that a given compound always contains the exact same proportion of elements by mass. This is known as the law of definite proportion. For example, all samples of water contain 88.8% oxygen by mass, and 11.2% hydrogen by mass. ...
Atoms - Mrs. Lindenlaub
... come upon a particle of copper that could no longer be divided and still have the chemical properties of copper. This final particle is an atom. ...
... come upon a particle of copper that could no longer be divided and still have the chemical properties of copper. This final particle is an atom. ...
2.Carbohydrates - Distance Education Chennai
... cellular components such as proteins, carbohydrates, lipids, nucleic acids and other biomolecules —although increasingly processes rather than individual molecules are the main focus. Among the vast number of different biomolecules, many are complex and large molecules (called biopolymers), which ar ...
... cellular components such as proteins, carbohydrates, lipids, nucleic acids and other biomolecules —although increasingly processes rather than individual molecules are the main focus. Among the vast number of different biomolecules, many are complex and large molecules (called biopolymers), which ar ...
BCH 101 - KSU Faculty Member websites
... and β) of the hemiacetals and hemiketals. The carbon about which this rotation occurs is the anomeric carbon and the two forms are termed anomers. Carbohydrates can change spontaneously between the α and β configurations: a process known as mutarotation. When drawn in the Fischer projection, the α c ...
... and β) of the hemiacetals and hemiketals. The carbon about which this rotation occurs is the anomeric carbon and the two forms are termed anomers. Carbohydrates can change spontaneously between the α and β configurations: a process known as mutarotation. When drawn in the Fischer projection, the α c ...
ch02 lecture 7e
... Sample Problem 2.1 SOLUTION: Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. ...
... Sample Problem 2.1 SOLUTION: Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. ...
sec 3- Counting atoms - Nutley Public Schools
... • The average atomic mass of copper can be calculated by multiplying the atomic mass of each isotope by its relative abundance (expressed in decimal form) and adding the results. ...
... • The average atomic mass of copper can be calculated by multiplying the atomic mass of each isotope by its relative abundance (expressed in decimal form) and adding the results. ...
How to Balance Chemical Equations
... involved are set and their formulas can not be altered. Hence, any change to the subscripts is NOT allowed. ONLY COEFFICIENTS ARE ALLOWED TO BE CHANGED!! ...
... involved are set and their formulas can not be altered. Hence, any change to the subscripts is NOT allowed. ONLY COEFFICIENTS ARE ALLOWED TO BE CHANGED!! ...
chemical reaction
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
sec 3- Counting atoms - Nutley Public Schools
... • The average atomic mass of copper can be calculated by multiplying the atomic mass of each isotope by its relative abundance (expressed in decimal form) and adding the results. ...
... • The average atomic mass of copper can be calculated by multiplying the atomic mass of each isotope by its relative abundance (expressed in decimal form) and adding the results. ...
halogen compounds organic chemistry
... chloride or cuprous bromide dissolved in corresponding halogen acids. Chloro and bromoarenes are formed. Diazonium salts required for this purpose are prepared by treating ice-cold solution of aniline in excess of dilute HCl with an aqueous solution of sodium nitrite at low temperature (0-5oC). This ...
... chloride or cuprous bromide dissolved in corresponding halogen acids. Chloro and bromoarenes are formed. Diazonium salts required for this purpose are prepared by treating ice-cold solution of aniline in excess of dilute HCl with an aqueous solution of sodium nitrite at low temperature (0-5oC). This ...
Counting Atoms
... • The average atomic mass of copper can be calculated by multiplying the atomic mass of each isotope by its relative abundance (expressed in decimal form) and adding the results. ...
... • The average atomic mass of copper can be calculated by multiplying the atomic mass of each isotope by its relative abundance (expressed in decimal form) and adding the results. ...
Chemistry: A Molecular Approach
... oxygen for every 1.00 g of carbon carbon dioxide contains 2.67 g of oxygen for every 1.00 g of carbon since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon diox ...
... oxygen for every 1.00 g of carbon carbon dioxide contains 2.67 g of oxygen for every 1.00 g of carbon since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon diox ...
The Big book of C1 chemistry
... In an atom, the number of electrons is equal to the number of protons in the nucleus. Atoms have no overall electrical charge. All atoms of a particular element have the same number of protons. Atoms of different elements have different numbers of protons. The number of protons in an atom of an elem ...
... In an atom, the number of electrons is equal to the number of protons in the nucleus. Atoms have no overall electrical charge. All atoms of a particular element have the same number of protons. Atoms of different elements have different numbers of protons. The number of protons in an atom of an elem ...
What is Chemistry? Chemistry
... How can we determine the charge of an ion? o For some of the elements it is very easy. Elements in groups 1, 2, 13, 14, 15, 16, & 17 will lose or gain electrons so they have the same # as the nearest _________________________________________ o The _______________________________________________ cann ...
... How can we determine the charge of an ion? o For some of the elements it is very easy. Elements in groups 1, 2, 13, 14, 15, 16, & 17 will lose or gain electrons so they have the same # as the nearest _________________________________________ o The _______________________________________________ cann ...
chemical reaction - MRS. STOTTS CHEMISTRY
... A chemical reaction is the process by which one or more substances are changed into one or more different substances. • the original substances are known as the reactants • the resulting substances are known as the products. According to the law of conservation of mass, the total mass of reactants m ...
... A chemical reaction is the process by which one or more substances are changed into one or more different substances. • the original substances are known as the reactants • the resulting substances are known as the products. According to the law of conservation of mass, the total mass of reactants m ...
Atomic Structure Practice Test Multiple Choice Identify the choice
... a. he saw the whole spectrum of colors. c. he saw electrons jumping to higher levels. b. he saw narrow bands of a few colors. d. he saw white light. ____ 15. Thomson’s experiments with cathode rays led to the discovery of the a. proton b. nucleus. c. neutron. ...
... a. he saw the whole spectrum of colors. c. he saw electrons jumping to higher levels. b. he saw narrow bands of a few colors. d. he saw white light. ____ 15. Thomson’s experiments with cathode rays led to the discovery of the a. proton b. nucleus. c. neutron. ...
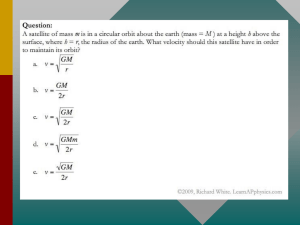
Question paper - Unit G485 - Fields, particles and frontiers of
... described in (b). Describe and explain the effect on the deflection of the ions after they have travelled between the plates using the same electric and magnetic fields of (c). ...
... described in (b). Describe and explain the effect on the deflection of the ions after they have travelled between the plates using the same electric and magnetic fields of (c). ...
Atomic Structure Practice Test
... a. he saw the whole spectrum of colors. c. he saw electrons jumping to higher levels. b. he saw narrow bands of a few colors. d. he saw white light. ____ 15. Thomson’s experiments with cathode rays led to the discovery of the a. proton b. nucleus. c. neutron. ...
... a. he saw the whole spectrum of colors. c. he saw electrons jumping to higher levels. b. he saw narrow bands of a few colors. d. he saw white light. ____ 15. Thomson’s experiments with cathode rays led to the discovery of the a. proton b. nucleus. c. neutron. ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.