FUNCTIONALIZATION OF NON-ACTiVATED CARBON ATOMS
... ratio and also the thermodynamical parameters. As expected, the 1413isomer is the most stable one; the ratio 1413H/l4czH is equal to 8 and that of 513H/5cxH to 6". The corresponding ratio in methylhydrindane is 200and in methyldecalin is 2.3. This difference can be accounted for by a simple conforma ...
... ratio and also the thermodynamical parameters. As expected, the 1413isomer is the most stable one; the ratio 1413H/l4czH is equal to 8 and that of 513H/5cxH to 6". The corresponding ratio in methylhydrindane is 200and in methyldecalin is 2.3. This difference can be accounted for by a simple conforma ...
CHEMICAL REACTIONS Chapter 4
... Depict the kind of reactants and products and their relative amounts in a reaction. ...
... Depict the kind of reactants and products and their relative amounts in a reaction. ...
Chapter 1 (Matter and Measurement) Objectives
... Understand that regular, repeatable patterns occur in the periodic table Appreciate that these patterns sometimes have notable exceptions Explain how the periodic law can be used to predict physical and chemical properties of elements Recall and understand that the noble gases have full outer shells ...
... Understand that regular, repeatable patterns occur in the periodic table Appreciate that these patterns sometimes have notable exceptions Explain how the periodic law can be used to predict physical and chemical properties of elements Recall and understand that the noble gases have full outer shells ...
Slide 1
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
Dear Students, Welcome to AP Chemistry, a little early. We will have
... a. Element: substance that cannot be broken down into other simpler substances by chemical reactions: substances composed of one type of atom; represented by a chemical symbol h. Compound: substance made up of atoms of two or more elements that combine in fixed propoltions or definite ratios: re ...
... a. Element: substance that cannot be broken down into other simpler substances by chemical reactions: substances composed of one type of atom; represented by a chemical symbol h. Compound: substance made up of atoms of two or more elements that combine in fixed propoltions or definite ratios: re ...
Chapter02_LEC - Mr. Fischer.com
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
TRO Chapter 2
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
Chemistry: A Molecular Approach
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
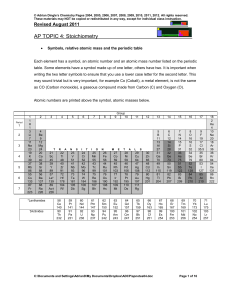
Topic #4 Notes
... 2. A hydrocarbon (a compound containing only hydrogen & carbon) is found to be 7.690% H and 92.31% C by mass. Calculate its empirical formula. ...
... 2. A hydrocarbon (a compound containing only hydrogen & carbon) is found to be 7.690% H and 92.31% C by mass. Calculate its empirical formula. ...
Solutions - Dynamic Science
... pattern (singlet) is shifted well over to the left. Notice the difference in the NMR spectra of both isomers. In the NMR spectrum of ethyl ethanoate the splitting pattern (quartet) of the CH2 next to the oxygen is shifted far to the left. ...
... pattern (singlet) is shifted well over to the left. Notice the difference in the NMR spectra of both isomers. In the NMR spectrum of ethyl ethanoate the splitting pattern (quartet) of the CH2 next to the oxygen is shifted far to the left. ...
1 FORMATION OF THE ATOMIC THEORY
... atoms. Hence, there should be a limitation in the number of types of atoms. Dalton’s atomic theory requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his ...
... atoms. Hence, there should be a limitation in the number of types of atoms. Dalton’s atomic theory requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his ...
Atom
... Calculating Atomic Mass Element X has two naturally occurring isotopes. The isotope with a mass of 10.012 amu (10X) has a relative abundance of 19.91 percent. The isotope with a mass of 11.009 amu (11X) has a relative abundance of 80.09 percent. Calculate the atomic mass of element X. ...
... Calculating Atomic Mass Element X has two naturally occurring isotopes. The isotope with a mass of 10.012 amu (10X) has a relative abundance of 19.91 percent. The isotope with a mass of 11.009 amu (11X) has a relative abundance of 80.09 percent. Calculate the atomic mass of element X. ...
4.3 Distinguishing Among Atoms
... 4.3 Distinguishing Among Atoms > Atomic Mass Carbon has two stable isotopes: carbon-12, which has a natural abundance of 98.89 percent, and carbon-13, which has a natural abundance of 1.11 percent. • The mass of carbon-12 is 12.000 amu; the mass of carbon-13 is 13.003 amu. • The atomic mass of carb ...
... 4.3 Distinguishing Among Atoms > Atomic Mass Carbon has two stable isotopes: carbon-12, which has a natural abundance of 98.89 percent, and carbon-13, which has a natural abundance of 1.11 percent. • The mass of carbon-12 is 12.000 amu; the mass of carbon-13 is 13.003 amu. • The atomic mass of carb ...
4.3 Distinguishing Among Atoms - Miami Beach Senior High School
... 4.3 Distinguishing Among Atoms > Atomic Mass Carbon has two stable isotopes: carbon-12, which has a natural abundance of 98.89 percent, and carbon-13, which has a natural abundance of 1.11 percent. • The mass of carbon-12 is 12.000 amu; the mass of carbon-13 is 13.003 amu. • The atomic mass of carb ...
... 4.3 Distinguishing Among Atoms > Atomic Mass Carbon has two stable isotopes: carbon-12, which has a natural abundance of 98.89 percent, and carbon-13, which has a natural abundance of 1.11 percent. • The mass of carbon-12 is 12.000 amu; the mass of carbon-13 is 13.003 amu. • The atomic mass of carb ...
Document
... 4.3 Distinguishing Among Atoms > Atomic Mass Carbon has two stable isotopes: carbon-12, which has a natural abundance of 98.89 percent, and carbon-13, which has a natural abundance of 1.11 percent. • The mass of carbon-12 is 12.000 amu; the mass of carbon-13 is 13.003 amu. • The atomic mass of carb ...
... 4.3 Distinguishing Among Atoms > Atomic Mass Carbon has two stable isotopes: carbon-12, which has a natural abundance of 98.89 percent, and carbon-13, which has a natural abundance of 1.11 percent. • The mass of carbon-12 is 12.000 amu; the mass of carbon-13 is 13.003 amu. • The atomic mass of carb ...
Chapter 3
... compounds are made of the same 2 elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole ...
... compounds are made of the same 2 elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole ...
M - Science Skool!
... Amount of Substance, Moles and Avogadro's Constant Chemists need to know the amount of each substance in a chemical reaction. This lets them calculate just how much of each reactant they need, and how much useful product is likely to be made. In everyday life, the amount of something is usually its ...
... Amount of Substance, Moles and Avogadro's Constant Chemists need to know the amount of each substance in a chemical reaction. This lets them calculate just how much of each reactant they need, and how much useful product is likely to be made. In everyday life, the amount of something is usually its ...
Isotope shift in the electron affinity of chlorine
... lected in a sector magnet and focused by means of several Einzel lenses. In an analyzing chamber, the ions were merged with a laser beam between two electrostatic quadrupole deflectors placed 50 cm apart. An ion current of typically 6 nA for 35 Cl and 2 nA for 37 Cl was measured with a Faraday cup ...
... lected in a sector magnet and focused by means of several Einzel lenses. In an analyzing chamber, the ions were merged with a laser beam between two electrostatic quadrupole deflectors placed 50 cm apart. An ion current of typically 6 nA for 35 Cl and 2 nA for 37 Cl was measured with a Faraday cup ...
chemical reaction
... • 1. In your own words, write a definition for each of the following terms: synthesis reaction and decomposition reaction. • 2. What type of reaction does the following equation represent? FeS + 2HCl -> FeCl2 +H2S ...
... • 1. In your own words, write a definition for each of the following terms: synthesis reaction and decomposition reaction. • 2. What type of reaction does the following equation represent? FeS + 2HCl -> FeCl2 +H2S ...
The Mole: A Shortcut for Chemists
... • The mole is a counting unit for chemists, the same way a baker uses a dozen. • 1 dozen = 12 objects • 1 mole = 6.02 × 1023 objects = 602,000,000,000,000,000,000,000 objects • That’s almost a trillion trillion! • 6.02 × 1023 is called Avogadro’s number. • “Mole” in writing; “mol” in calculations. ...
... • The mole is a counting unit for chemists, the same way a baker uses a dozen. • 1 dozen = 12 objects • 1 mole = 6.02 × 1023 objects = 602,000,000,000,000,000,000,000 objects • That’s almost a trillion trillion! • 6.02 × 1023 is called Avogadro’s number. • “Mole” in writing; “mol” in calculations. ...
National 5 Chemistry Unit 3 Chemistry In Society
... d) Condensation polymerisation Condensation polymerisation is a process whereby many small monomer molecules join together to form one large polymer, with water, or some other small molecule formed at the same time. The monomers have more than one functional group. ...
... d) Condensation polymerisation Condensation polymerisation is a process whereby many small monomer molecules join together to form one large polymer, with water, or some other small molecule formed at the same time. The monomers have more than one functional group. ...
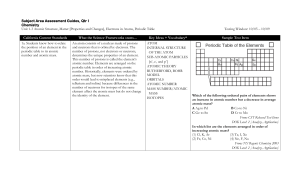
Subject Area Assessment Guides
... table. The transition metals (Groups 3 through 12) are represented by some of the most common metals, such as iron, copper, gold, mercury, silver, and zinc. All these elements have electrons in their outer d orbitals. Electronegativity is a measure of the ability of an atom of an element to attract ...
... table. The transition metals (Groups 3 through 12) are represented by some of the most common metals, such as iron, copper, gold, mercury, silver, and zinc. All these elements have electrons in their outer d orbitals. Electronegativity is a measure of the ability of an atom of an element to attract ...
problems - chem.msu.su
... 3. What factors affect the solubility of K3[Co(NO2)6] in the mother solution after precipitate formation? Choose the right answers: a) stability constant of complex ion [Co(NO2)6]3–; b) solubility product of the precipitate; c) concentration of K+; d) concentration of Co(II); e) concentration of NO2 ...
... 3. What factors affect the solubility of K3[Co(NO2)6] in the mother solution after precipitate formation? Choose the right answers: a) stability constant of complex ion [Co(NO2)6]3–; b) solubility product of the precipitate; c) concentration of K+; d) concentration of Co(II); e) concentration of NO2 ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.