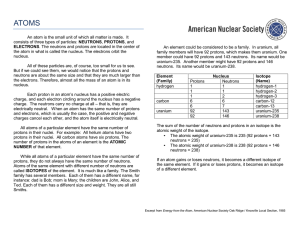
An atom is the small unit of which all matter is made. It consists of
... the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size and that they are much larger than the electrons. Therefore, almost ...
... the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size and that they are much larger than the electrons. Therefore, almost ...
Chemical Equations and Reactions
... • the more an element reacts with other substances, the greater the activity is. • Metals: the greater the activity, the greater it loses electrons (to form cations) • Non-metals: the greater the activity, the greater it gains electrons (to form anions) • Activity series: a list of which elements a ...
... • the more an element reacts with other substances, the greater the activity is. • Metals: the greater the activity, the greater it loses electrons (to form cations) • Non-metals: the greater the activity, the greater it gains electrons (to form anions) • Activity series: a list of which elements a ...
Unit 1 PowerPoint Complete Notes
... following are hints to help determine the physical state. (s) – most metals, precipitates (l) – mercury, bromine, water (g) – noble gases, diatomic molecules (except bromine), ammonia (aq) – substance is in a water based solution (use solubility chart) ...
... following are hints to help determine the physical state. (s) – most metals, precipitates (l) – mercury, bromine, water (g) – noble gases, diatomic molecules (except bromine), ammonia (aq) – substance is in a water based solution (use solubility chart) ...
neutrons
... number due to varying numbers of neutrons Isotopes are usually identified by specifying their mass number. Two methods for specifying isotopes: The mass number is written with a hyphen after the name of the element ex: hydrogen-3 is tritium Show the composition of a nucleus as the isotopes nucle ...
... number due to varying numbers of neutrons Isotopes are usually identified by specifying their mass number. Two methods for specifying isotopes: The mass number is written with a hyphen after the name of the element ex: hydrogen-3 is tritium Show the composition of a nucleus as the isotopes nucle ...
Matter- Types and Changes
... each other. These are referred to as diatomic or polyatomic molecules. H2, O2, N2, F2, Cl2, Br2, I2 C60, S8, P4 • The 2 in O2 is termed a subscript and refers to the element immediately in front of it. ...
... each other. These are referred to as diatomic or polyatomic molecules. H2, O2, N2, F2, Cl2, Br2, I2 C60, S8, P4 • The 2 in O2 is termed a subscript and refers to the element immediately in front of it. ...
Sample Exam 2 Questions
... 16. In chemiosmotic synthesis of ATP during photosynthesis, the hydrogen ions diffuse from the A. stroma to the thylakoid interior. B. cytoplasm to cross the outer chloroplast membrane. C. stroma to cross the inner chloroplast membrane. D. thylakoid interior to the stroma. E. space between the inne ...
... 16. In chemiosmotic synthesis of ATP during photosynthesis, the hydrogen ions diffuse from the A. stroma to the thylakoid interior. B. cytoplasm to cross the outer chloroplast membrane. C. stroma to cross the inner chloroplast membrane. D. thylakoid interior to the stroma. E. space between the inne ...
Review of Moles and Stoichiometry
... 15.) A compound was analyzed in a lab to determine its empirical formula. Decomposition of the compound at standard temperature and pressure produced 9.00 g carbon, 16.8 L hydrogen, and 2.80 L oxygen. a.) What is the empirical formula for this compound? ...
... 15.) A compound was analyzed in a lab to determine its empirical formula. Decomposition of the compound at standard temperature and pressure produced 9.00 g carbon, 16.8 L hydrogen, and 2.80 L oxygen. a.) What is the empirical formula for this compound? ...
Nuclear Radiation1516
... Spontaneous emission of particles (alpha, beta, neutron) or radiation (gamma), or both at the same time, from the decay of certain radioisotopes. Isotopes of some elements are radioactive, especially the large elements. Radioactive elements are unstable and will undergo radioactive decay. ...
... Spontaneous emission of particles (alpha, beta, neutron) or radiation (gamma), or both at the same time, from the decay of certain radioisotopes. Isotopes of some elements are radioactive, especially the large elements. Radioactive elements are unstable and will undergo radioactive decay. ...
Atoms, Ions and Molecules
... is one square of pure colour). A compound is like one of the image pixels that make up the picture. An element is like one of the screen pixels that make up the image pixels — there are ...
... is one square of pure colour). A compound is like one of the image pixels that make up the picture. An element is like one of the screen pixels that make up the image pixels — there are ...
Atomic Theory
... All elements are composed of atoms Atoms of the same element are identical Atoms can physically mix or chemically combine in simple whole number ratios ...
... All elements are composed of atoms Atoms of the same element are identical Atoms can physically mix or chemically combine in simple whole number ratios ...
Study Guide –Chapter 4 Atomic Theory and The Atom
... 36. Protons stay together in the nucleus because of______________________. 37. Objects are pulled toward one another because of ______________________. 38. An important force in radioactive atoms is ______________________. 39. The electrons are held around the nucleus because of ____________________ ...
... 36. Protons stay together in the nucleus because of______________________. 37. Objects are pulled toward one another because of ______________________. 38. An important force in radioactive atoms is ______________________. 39. The electrons are held around the nucleus because of ____________________ ...
The Atom - Humble ISD
... • Help make up the nucleus of the atom • Help identify the atom (could be considered an atom’s DNA) • Equal to the atomic number of the atom • Contribute to the ...
... • Help make up the nucleus of the atom • Help identify the atom (could be considered an atom’s DNA) • Equal to the atomic number of the atom • Contribute to the ...
Physical Science
... mixtures of isotopes. Isotopes are atoms of the same element that differ in the number of neutrons. ...
... mixtures of isotopes. Isotopes are atoms of the same element that differ in the number of neutrons. ...
No Slide Title
... Mass Number (atomic mass)- ( P + N) This is the number of protons and neutrons in the nucleus. Isotope - This is an atom of an element where the number of neutrons have changed. ( Ex: hydrogen isotopes) Go to Section: ...
... Mass Number (atomic mass)- ( P + N) This is the number of protons and neutrons in the nucleus. Isotope - This is an atom of an element where the number of neutrons have changed. ( Ex: hydrogen isotopes) Go to Section: ...
Atomic Structure
... nuclei” Why? • Beta particles are high speed electrons • Gamma radiation is a form of electromagnetic radiation. ...
... nuclei” Why? • Beta particles are high speed electrons • Gamma radiation is a form of electromagnetic radiation. ...
File - Mr. Walsh`s AP Chemistry
... named by describing the molecular formula, using prefixes for the numbers. o You will need to memorize the number prefixes for the numbers 1–10. o E.g., P2O5 is diphosphorus pentoxide. **Note that the prefix “mono—“ is never used with the first element. SO3 is simply sulfur trioxide. However, “mono— ...
... named by describing the molecular formula, using prefixes for the numbers. o You will need to memorize the number prefixes for the numbers 1–10. o E.g., P2O5 is diphosphorus pentoxide. **Note that the prefix “mono—“ is never used with the first element. SO3 is simply sulfur trioxide. However, “mono— ...
2 – Atomic Structure - Science at St. Dominics
... of elements from isotopic abundance 1. Find out the percentage abundance of each isotope of the element. You are usually given this information. 2. Pretend you have 100 atoms of that element. 3. Calculate the mass of 100 atoms from their isotopic abundance. 4. Divide by 100 to get the relative atomi ...
... of elements from isotopic abundance 1. Find out the percentage abundance of each isotope of the element. You are usually given this information. 2. Pretend you have 100 atoms of that element. 3. Calculate the mass of 100 atoms from their isotopic abundance. 4. Divide by 100 to get the relative atomi ...
1.1 to 1.4
... that contains two or more kinds of atoms in fixed proportions. Ex. H2O • can be broken down • can be either ionic (made up of a metal and a nonmetal) or molecular (made up of two or more non- ...
... that contains two or more kinds of atoms in fixed proportions. Ex. H2O • can be broken down • can be either ionic (made up of a metal and a nonmetal) or molecular (made up of two or more non- ...
Chemistry Lecture No.4______By : Asst. Lect. Tariq-H-AL
... Even at very low levels of exposure, there is danger from radiation. For example, x rays used in diagnosis are not completely free of potential harm to a patient. Persons who administer x-rays must take precautions to avoid exposure, because the effect is cumulative. Exposure to high levels of radia ...
... Even at very low levels of exposure, there is danger from radiation. For example, x rays used in diagnosis are not completely free of potential harm to a patient. Persons who administer x-rays must take precautions to avoid exposure, because the effect is cumulative. Exposure to high levels of radia ...
Name
... Very few positively charged alpha particles deflected revealing a tiny, dense, positive region in atoms. 17. C 18. B 19. D 20. They are isotopes b/c they have different numbers of neutrons, but they are the same element b/c they have the same number of protons (same atomic number). 21. D 22. C 23. A ...
... Very few positively charged alpha particles deflected revealing a tiny, dense, positive region in atoms. 17. C 18. B 19. D 20. They are isotopes b/c they have different numbers of neutrons, but they are the same element b/c they have the same number of protons (same atomic number). 21. D 22. C 23. A ...
lecture 13
... Balancing chemical equations is an application of both the Modern Atomic Theory and the Law of Conservation of Mass. BALANCING EQUATIONS: The same number of each type of element must occur on the left (BEFORE the reaction) and on the right (AFTER the reaction) ...
... Balancing chemical equations is an application of both the Modern Atomic Theory and the Law of Conservation of Mass. BALANCING EQUATIONS: The same number of each type of element must occur on the left (BEFORE the reaction) and on the right (AFTER the reaction) ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.