Lecture 12 Slides
... Thermodynamics and Thermochemistry Thermodynamics -- the science of energy and its transformations Thermochemistry -- the branch of thermodynamics specifically focused on the changes in energy and transfer of heat related to ...
... Thermodynamics and Thermochemistry Thermodynamics -- the science of energy and its transformations Thermochemistry -- the branch of thermodynamics specifically focused on the changes in energy and transfer of heat related to ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... Calculate the equilibrium conversion of SO2 reacting with air at 25˚C and at 600˚Cfor a feed that contains 1) stoichiometric ratio of reactants 2) large excess of air. Assume constant heat or reaction. ...
... Calculate the equilibrium conversion of SO2 reacting with air at 25˚C and at 600˚Cfor a feed that contains 1) stoichiometric ratio of reactants 2) large excess of air. Assume constant heat or reaction. ...
Chem 480A
... (There is an interesting problem in going from Equation 5a to Equation 5b which is not usually discussed. In Equation 5a the coefficients of the balanced chemical equation, a, b, etc. have units of moles, so that, for example, cC is moles times Joules per mole which leaves just units of Joules. How ...
... (There is an interesting problem in going from Equation 5a to Equation 5b which is not usually discussed. In Equation 5a the coefficients of the balanced chemical equation, a, b, etc. have units of moles, so that, for example, cC is moles times Joules per mole which leaves just units of Joules. How ...
Theoretical Calculation of Enthalpy of reactions involved in PZ
... Combining the well-known Gibbs Helmholtz equation [3] with equations 8 and 9, the enthalpy of the overall reaction can be expressed as ...
... Combining the well-known Gibbs Helmholtz equation [3] with equations 8 and 9, the enthalpy of the overall reaction can be expressed as ...
THERMODYNAMICS
... Many forms of energy can be interconverted and that in chemical processes, chemical energy is converted to heat energy or vice versa. The amount of heat a process uses (endothermic) or gives off (exothermic) can tell us a great deal about that process. For this reason it is important for us to be ...
... Many forms of energy can be interconverted and that in chemical processes, chemical energy is converted to heat energy or vice versa. The amount of heat a process uses (endothermic) or gives off (exothermic) can tell us a great deal about that process. For this reason it is important for us to be ...
What is Thermodynamics?
... changes as a function of T and P. • Regardless of T and P, a reaction occurs spontaneously in the direction that will reduce the value of ∆GR because ∆GR is the driving energy for the chemical reaction. • For any T and P conditions, a reaction at equilibrium has ∆GR = 0 because when a reaction is at ...
... changes as a function of T and P. • Regardless of T and P, a reaction occurs spontaneously in the direction that will reduce the value of ∆GR because ∆GR is the driving energy for the chemical reaction. • For any T and P conditions, a reaction at equilibrium has ∆GR = 0 because when a reaction is at ...
(Thermochemistry-Chapter 5) - Fall 2015
... 1. In an experiment similar to the procedure set out for Part (A) of the Calorimetry experiment, 1.500 g of Mg(s) was combined with 125.0 mL of 1.0 M HCl. The initial temperature was 25.0oC and the final temperature was 72.3oC. Calculate: (a) the heat involved in the reaction and (b) the enthalpy of ...
... 1. In an experiment similar to the procedure set out for Part (A) of the Calorimetry experiment, 1.500 g of Mg(s) was combined with 125.0 mL of 1.0 M HCl. The initial temperature was 25.0oC and the final temperature was 72.3oC. Calculate: (a) the heat involved in the reaction and (b) the enthalpy of ...
Thermochemistry ppt with inkings
... 1. In an experiment similar to the procedure set out for Part (A) of the Calorimetry experiment, 1.500 g of Mg(s) was combined with 125.0 mL of 1.0 M HCl. The initial temperature was 25.0oC and the final temperature was 72.3oC. Calculate: (a) the heat involved in the reaction and (b) the enthalpy of ...
... 1. In an experiment similar to the procedure set out for Part (A) of the Calorimetry experiment, 1.500 g of Mg(s) was combined with 125.0 mL of 1.0 M HCl. The initial temperature was 25.0oC and the final temperature was 72.3oC. Calculate: (a) the heat involved in the reaction and (b) the enthalpy of ...
Thermochemistry - Valdosta State University
... _________________ – a process that is determined by its initial and final conditions. • “A process that is ______________________.” • ______ (w) and _____ (q) _______ state functions. • Energy change (____) ______ a state function. ...
... _________________ – a process that is determined by its initial and final conditions. • “A process that is ______________________.” • ______ (w) and _____ (q) _______ state functions. • Energy change (____) ______ a state function. ...
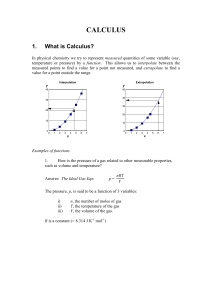
CALCULUS
... How is the pressure of a gas related to other measurable properties, such as volume and temperature? Answer: The Ideal Gas Eqn. ...
... How is the pressure of a gas related to other measurable properties, such as volume and temperature? Answer: The Ideal Gas Eqn. ...
P2-Equilibrium Activity
... Not all chemical reactions reach completion where the limiting reactant is consumed completely. In fact, most chemical reactions that occur in living systems never reach completion. Rather, they produce some amount of product then appear to stop reacting in the forward direction, never fully consumi ...
... Not all chemical reactions reach completion where the limiting reactant is consumed completely. In fact, most chemical reactions that occur in living systems never reach completion. Rather, they produce some amount of product then appear to stop reacting in the forward direction, never fully consumi ...
Chapter 2 Guided Notes
... are exothermic processes. The law of conservation of _________ states that during any physical or chemical change, the total quantity of energy remains constant. In other words, energy cannot be destroyed or created. To keep track of energy changes, chemists use the terms system and surroundings. A ...
... are exothermic processes. The law of conservation of _________ states that during any physical or chemical change, the total quantity of energy remains constant. In other words, energy cannot be destroyed or created. To keep track of energy changes, chemists use the terms system and surroundings. A ...