Study Guide
... Calculate the rate of reactant consumption and product formation in reactions. Identify postulates of collision theory. Apply collision theory to explain reaction rates and factors affecting reaction rates. Explain how surface area, nature of reactants, concentration, temperature and catalysts which ...
... Calculate the rate of reactant consumption and product formation in reactions. Identify postulates of collision theory. Apply collision theory to explain reaction rates and factors affecting reaction rates. Explain how surface area, nature of reactants, concentration, temperature and catalysts which ...
Sample pages 2 PDF
... The traditional thermometers consist of a glass bulb containing a liquid connected to a capillary tube several centimeters long. When in contact with a warmer body, the liquid expands; the higher the temperature, the higher it rises in the capillary. Mercury was in standard use as thermometer liquid ...
... The traditional thermometers consist of a glass bulb containing a liquid connected to a capillary tube several centimeters long. When in contact with a warmer body, the liquid expands; the higher the temperature, the higher it rises in the capillary. Mercury was in standard use as thermometer liquid ...
Valence bond and Molecular orbital theory for diatomic
... 3. “Organic Chemistry”, R. T. Morrison and R. N. Boyd, 6th Edition (1992), Prentice-Hall of India (P) Ltd., New Delhi. 4. “Organic Chemistry”, S. M. Mukherjee, S. P. Singh, and R. P. Kapoor, 1st Edition (1985), New Age International (P) Ltd. Publishers, New Delhi. 5. “Principles of Physical Chemistr ...
... 3. “Organic Chemistry”, R. T. Morrison and R. N. Boyd, 6th Edition (1992), Prentice-Hall of India (P) Ltd., New Delhi. 4. “Organic Chemistry”, S. M. Mukherjee, S. P. Singh, and R. P. Kapoor, 1st Edition (1985), New Age International (P) Ltd. Publishers, New Delhi. 5. “Principles of Physical Chemistr ...
WM White Geochemistry Chapter 2: Fundamental - U
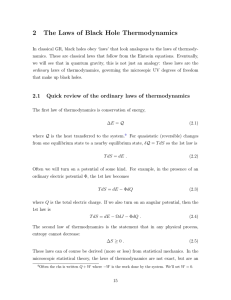
... e defined geochemistry as the application of chemical knowledge and techniques to solve geological problems. It is appropriate, then, to begin our study of geochemistry with a review of physical chemistry. Our initial focus will be on thermodynamics. Strictly defined, thermodynamics is the study of ...
... e defined geochemistry as the application of chemical knowledge and techniques to solve geological problems. It is appropriate, then, to begin our study of geochemistry with a review of physical chemistry. Our initial focus will be on thermodynamics. Strictly defined, thermodynamics is the study of ...
Equilibrium Review Problems N2(g) + H2(g) NH3(g) 1. When 3.29
... C(s) + H2O(g) CO(g) + H2(g) Hº = +131kJ A rigid container holds a mixture of graphite pellets (C(s)), H2O(g), CO(g), and H2(g) at equilibrium. State whether the number of moles of CO(g) in the container will increase, decrease, or remain the same after each of the following disturbances is applie ...
... C(s) + H2O(g) CO(g) + H2(g) Hº = +131kJ A rigid container holds a mixture of graphite pellets (C(s)), H2O(g), CO(g), and H2(g) at equilibrium. State whether the number of moles of CO(g) in the container will increase, decrease, or remain the same after each of the following disturbances is applie ...
Review_WB_1
... 2) A toy balloon has an internal pressure of 1.05 atm. If the temperature where the balloon is released is 20 0 C, what will the temperature have to be when the balloon raises to an altitude where the pressure is 0.85 atm? P ...
... 2) A toy balloon has an internal pressure of 1.05 atm. If the temperature where the balloon is released is 20 0 C, what will the temperature have to be when the balloon raises to an altitude where the pressure is 0.85 atm? P ...
CP Chemistry Final Review – Chap. 10-19
... 2. The side of a manometer open to the atmosphere is 100 mm higher than the side open to a gas sample. Assuming that atmospheric pressure 780 mm Hg, determine the pressure of the gas sample. 3. The gas pressure in a 20-L tank is 4.8 atm. What is the new pressure if the temp. is raised from 100°C to ...
... 2. The side of a manometer open to the atmosphere is 100 mm higher than the side open to a gas sample. Assuming that atmospheric pressure 780 mm Hg, determine the pressure of the gas sample. 3. The gas pressure in a 20-L tank is 4.8 atm. What is the new pressure if the temp. is raised from 100°C to ...
General Equilibrium FR worksheet
... When H2(g) is mixed with CO2(g) at 2,000 K, equilibrium is achieved according to the equation above. In one experiment, the following equilibrium concentrations were measured. [H2] = 0.20 mol/L [CO2] = 0.30 mol/L [H2O] = [CO] = 0.55 mol/L (a) What is the mole fraction of CO(g) in the equilibrium mix ...
... When H2(g) is mixed with CO2(g) at 2,000 K, equilibrium is achieved according to the equation above. In one experiment, the following equilibrium concentrations were measured. [H2] = 0.20 mol/L [CO2] = 0.30 mol/L [H2O] = [CO] = 0.55 mol/L (a) What is the mole fraction of CO(g) in the equilibrium mix ...
Thermochemistry - Moorpark College
... Laws of thermodynamics are useful in predicting outcomes, but unlike a theory do not explain the expected behavior. Laws of Thermodynamics: Zeroth Law: Temperature Concept: Temperature measures the intensity of hotness or coldness of an object. When two objects are brought together heat always flows ...
... Laws of thermodynamics are useful in predicting outcomes, but unlike a theory do not explain the expected behavior. Laws of Thermodynamics: Zeroth Law: Temperature Concept: Temperature measures the intensity of hotness or coldness of an object. When two objects are brought together heat always flows ...
Thermodynamics
... (Homework) 2 moles of a certain ideal gas is allowed to expand adiabatically and reversibly to 5 atm pressure from an initial state of 20°C and 15 atm. What will be the final temperature and volume of the gas? What is the change in internal energy during this process? Assume a Cp of 8.58 cal/mole K ...
... (Homework) 2 moles of a certain ideal gas is allowed to expand adiabatically and reversibly to 5 atm pressure from an initial state of 20°C and 15 atm. What will be the final temperature and volume of the gas? What is the change in internal energy during this process? Assume a Cp of 8.58 cal/mole K ...
Lecture19
... An air conditioner uses an amount of electrical energy U to cool a home. The amount of heat removed from the home must be less than or equal to U. ...
... An air conditioner uses an amount of electrical energy U to cool a home. The amount of heat removed from the home must be less than or equal to U. ...