Thermodynamic Properties of Hydrated and Ammoniated Electrons
... computed the energies of solvated electrons. They state that the total energy, Et, is the sum of the electronic energy, E,, and of the medium rearrangement of energy E M . ...
... computed the energies of solvated electrons. They state that the total energy, Et, is the sum of the electronic energy, E,, and of the medium rearrangement of energy E M . ...
2014
... observed in the container as the reaction reaches equilibrium? (A)A decrease in the strength of the intermolecular attractions among molecules in the container (B)An increase in the strength of the intermolecular attractions among molecules in the container (C)An increase in the number of molecules, ...
... observed in the container as the reaction reaches equilibrium? (A)A decrease in the strength of the intermolecular attractions among molecules in the container (B)An increase in the strength of the intermolecular attractions among molecules in the container (C)An increase in the number of molecules, ...
Equilibrium (PowerPoint) West Coast 2015
... Teaching Tidbit Context Our audience consists students in the second semester of a general chemistry course. Typical Units Unit 1 – Intermolecular forces and Solutions Unit 2 – Kinetics and Equilibrium Unit 3 – Thermodynamics ...
... Teaching Tidbit Context Our audience consists students in the second semester of a general chemistry course. Typical Units Unit 1 – Intermolecular forces and Solutions Unit 2 – Kinetics and Equilibrium Unit 3 – Thermodynamics ...
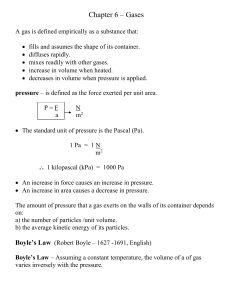
11-1 SECTION 11 THERMOCHEMISTRY Thermochemistry: Study of
... and bond formation. Bond breaking results in absorption of heat from the surroundings and bond formation in release of heat to the surroundings. Heat: Energy transferred as a result of a temperature difference between a system and its surroundings. The quantity of energy transferred from the surroun ...
... and bond formation. Bond breaking results in absorption of heat from the surroundings and bond formation in release of heat to the surroundings. Heat: Energy transferred as a result of a temperature difference between a system and its surroundings. The quantity of energy transferred from the surroun ...
Thermodynamics of Crystal-Melt Phase Change
... The enthalpy-temperature phase diagram, Fig. 2.1, shows that the equilibrium liquid and solid occupy distinct, non-overlapping regions within the H -T plane, with P fixed. Specifically, either solid or liquid exists as the preferred phase anywhere along their individual existence lines, each located ...
... The enthalpy-temperature phase diagram, Fig. 2.1, shows that the equilibrium liquid and solid occupy distinct, non-overlapping regions within the H -T plane, with P fixed. Specifically, either solid or liquid exists as the preferred phase anywhere along their individual existence lines, each located ...
Notes
... Does how we effect the transfer of heat and work matter to the value of the internal energy change? Why or why not? How can we measure heat flow? How is temperature change related to the transfer of heat? Does the composition of the substance that undergoes a change in temperature matter, matter if ...
... Does how we effect the transfer of heat and work matter to the value of the internal energy change? Why or why not? How can we measure heat flow? How is temperature change related to the transfer of heat? Does the composition of the substance that undergoes a change in temperature matter, matter if ...
Chem 11, Notes – Unit 3 – Properties of Matter
... • A hypothesis is a statement that is unproven that is yet to be tested • A theory is a set of hypotheses that come from observations from the natural world o A theory has been repeatedly tested and is widely accepted explains phenomena • A law will state the results of experiments and state what ...
... • A hypothesis is a statement that is unproven that is yet to be tested • A theory is a set of hypotheses that come from observations from the natural world o A theory has been repeatedly tested and is widely accepted explains phenomena • A law will state the results of experiments and state what ...
unit 4 practice
... 2. Equal volumes and concentrations of hydrochloric acid and ethanoic acid are titrated with sodium hydroxide solutions of the same concentration. Which statement is correct? A. The initial pH values of ...
... 2. Equal volumes and concentrations of hydrochloric acid and ethanoic acid are titrated with sodium hydroxide solutions of the same concentration. Which statement is correct? A. The initial pH values of ...
Cause of Chirality Consensus
... very important for the system to reach and maintain highentropy non-equilibrium partitions. In nature many powerful mechanisms associate with functional structures as is expressed by the catchphrase ‘structure-activity relationship’. When diverse mechanisms draw from the same limited resources, the ...
... very important for the system to reach and maintain highentropy non-equilibrium partitions. In nature many powerful mechanisms associate with functional structures as is expressed by the catchphrase ‘structure-activity relationship’. When diverse mechanisms draw from the same limited resources, the ...
Name: ______ Date
... a) A process that absorbs energy from its surroundings is called endothermic. b) In an exothermic reaction the enthalpy of species increases. c) Energy is the capacity to do work or to transfer heat. d) Kinetic energy is the energy of motion. There are two properties of a reacting system that determ ...
... a) A process that absorbs energy from its surroundings is called endothermic. b) In an exothermic reaction the enthalpy of species increases. c) Energy is the capacity to do work or to transfer heat. d) Kinetic energy is the energy of motion. There are two properties of a reacting system that determ ...
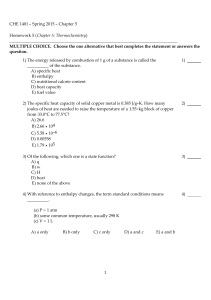
CHE 1401 - Spring 2015 - Chapter 5 Homework 5 (Chapter 5
... 14) Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to t ...
... 14) Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to t ...
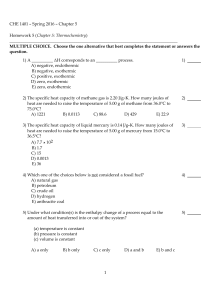
CHE 1401 - Spring 2016 - Chapter 5 Homework 5 (Chapter 5
... Calculate the value of q (kJ) in this exothermic reaction when 4.00 g of hydrogen peroxide decomposes at constant pressure? A) -0.0217 B) -11.5 C) -2.31 × 104 D) -23.1 E) 1.44 39) Which one of the following conditions would always result in an increase in the internal energy of a system? A) The syst ...
... Calculate the value of q (kJ) in this exothermic reaction when 4.00 g of hydrogen peroxide decomposes at constant pressure? A) -0.0217 B) -11.5 C) -2.31 × 104 D) -23.1 E) 1.44 39) Which one of the following conditions would always result in an increase in the internal energy of a system? A) The syst ...
CHE 1401 - Fall 2016 - Chapter 5 Homework 5 (Chapter 5
... is __________, and therefore heat is __________ by the reaction. A) exothermic, absorbed B) exothermic, released C) endothermic, released D) endothermic, absorbed E) thermoneutral, neither released nor absorbed 27) Which of the following is a statement of Hess's law? A) The ΔH for a process in the f ...
... is __________, and therefore heat is __________ by the reaction. A) exothermic, absorbed B) exothermic, released C) endothermic, released D) endothermic, absorbed E) thermoneutral, neither released nor absorbed 27) Which of the following is a statement of Hess's law? A) The ΔH for a process in the f ...