Notes 6 - Macdonald Research Group
... investigated classes of ligands since their discovery. NHCs are very basic and they are very strong nucleophiles. This makes them excellent donors that form stronger bonds to transition metals than ligands such as phosphines. The adducts that they make are generally best considered as Fischer carben ...
... investigated classes of ligands since their discovery. NHCs are very basic and they are very strong nucleophiles. This makes them excellent donors that form stronger bonds to transition metals than ligands such as phosphines. The adducts that they make are generally best considered as Fischer carben ...
doc CHEM 222 Lab exam with Answers
... temperature and then allowing them to come back out of solution. 2.__T___ The purpose of refluxing is to carry out a reaction at the boiling point of the solvent. 3.__F___ All chemical reactions must take place in solution. 4.__T___ When a carbene is formed in the presence of an alkene, a cyclopropa ...
... temperature and then allowing them to come back out of solution. 2.__T___ The purpose of refluxing is to carry out a reaction at the boiling point of the solvent. 3.__F___ All chemical reactions must take place in solution. 4.__T___ When a carbene is formed in the presence of an alkene, a cyclopropa ...
Applications of N-Heterocyclic Carbenes in Organic Reactions
... Phosphane Coordinated to metal atom NHC Coordinated to metal atom ...
... Phosphane Coordinated to metal atom NHC Coordinated to metal atom ...
Slide 1
... They have virtually no p-acceptor ability unless a p-system is present. Increasing the carbon substitution (replacing hydrogens with hydrocarbon groups such as methyl, ethyl, isopropyl) usually increases the donor strength, but steric factors can come into play and weaken the metal-alkyl bond (e.g., ...
... They have virtually no p-acceptor ability unless a p-system is present. Increasing the carbon substitution (replacing hydrogens with hydrocarbon groups such as methyl, ethyl, isopropyl) usually increases the donor strength, but steric factors can come into play and weaken the metal-alkyl bond (e.g., ...
ligand design - UZH - Department of Chemistry
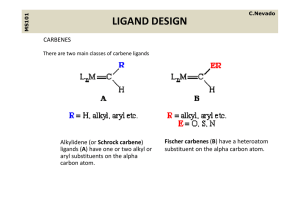
... the general formula (R2N)2C:, where the 'R's are various functional groups. ...
... the general formula (R2N)2C:, where the 'R's are various functional groups. ...
Metal Carbenes
... Carbenes are both thermodynamically and kinetically unstable therefore forming very strong metal-carbene bonds disfavoring dissociation e.g. just as pramagnetic triplet :CH2 can dimerize to form diamagnetic H2C=CH2, it also binds to a triplet LnM fragment to give a diamagnetic LnM=CH2 complex. ...
... Carbenes are both thermodynamically and kinetically unstable therefore forming very strong metal-carbene bonds disfavoring dissociation e.g. just as pramagnetic triplet :CH2 can dimerize to form diamagnetic H2C=CH2, it also binds to a triplet LnM fragment to give a diamagnetic LnM=CH2 complex. ...
Organometallic Chemistry between organic and inorganic
... • Many transition metals form not only M-C single bonds but also M=C and (more rare) even M≡C bonds. • Complexes containing an M=C bond are called carbene complexes – The ligand without the metal would be a free carbene ...
... • Many transition metals form not only M-C single bonds but also M=C and (more rare) even M≡C bonds. • Complexes containing an M=C bond are called carbene complexes – The ligand without the metal would be a free carbene ...
Organometallic Chemistry
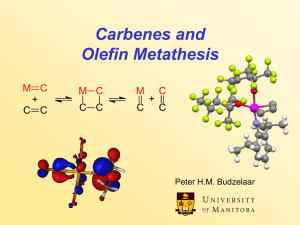
... symmetry forbidden and occur only photochemically. However, the presence of dorbitals on the metal alkylidene fragment breaks this symmetry and the reaction is quite facile. Normally, the products are statistical, unless the reaction can be driven in some way or the two alkenes have different reacti ...
... symmetry forbidden and occur only photochemically. However, the presence of dorbitals on the metal alkylidene fragment breaks this symmetry and the reaction is quite facile. Normally, the products are statistical, unless the reaction can be driven in some way or the two alkenes have different reacti ...
Slide 1
... In metal-free Arduengo carbenes interaction of empty p-orbital of the carbene carbon with filled p-orbitals of the adjacent atoms raises the energy of the former. It matches the energy of filled metal orbitals only poorly now and thus is a week acceptor . So, the metal-to-carbene bonding can be bett ...
... In metal-free Arduengo carbenes interaction of empty p-orbital of the carbene carbon with filled p-orbitals of the adjacent atoms raises the energy of the former. It matches the energy of filled metal orbitals only poorly now and thus is a week acceptor . So, the metal-to-carbene bonding can be bett ...
Document
... A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electron rich alkene. ...
... A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electron rich alkene. ...
Nugget
... Investigation of the Lewis Acidic Properties of N-Heterocyclic Carbene Ligands and Their Implications for Catalysis Colin D. Abernethy, Department of Chemistry, Keene State College Keene, NH 03435 Cyclopentadienyl complexes of vanadium chlorides, which also contain N-heterocyclic carbene (NHC) ligan ...
... Investigation of the Lewis Acidic Properties of N-Heterocyclic Carbene Ligands and Their Implications for Catalysis Colin D. Abernethy, Department of Chemistry, Keene State College Keene, NH 03435 Cyclopentadienyl complexes of vanadium chlorides, which also contain N-heterocyclic carbene (NHC) ligan ...
Carbenes and Nitrenes: Structure, generaNon and reacNvity
... Then, N-‐heterocyclic carbenes (NHCs) already seen. The first applica4ons of thiazolydenes in umpolung organocatalysis were reported as early as 1943 (J. Pharm. Soc. Jpn. 1943, 63, 296) and metal complexes of NHC ...
... Then, N-‐heterocyclic carbenes (NHCs) already seen. The first applica4ons of thiazolydenes in umpolung organocatalysis were reported as early as 1943 (J. Pharm. Soc. Jpn. 1943, 63, 296) and metal complexes of NHC ...
Persistent carbene

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the 'R's are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.Traditionally carbenes are viewed as so reactive that were only studied indirectly, e.g. by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, i.e., undergoing dimerization, many can be isolated as pure substances.Persistent carbenes can exist in the singlet or the triplet states with the singlet state carbenes being more stable. The relative stability of these compounds is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable in the absence of moisture and (in most cases) oxygen, and can be isolated and indefinitely stored. Others are not thermodynamically stable and will dimerise slowly over days. The less stable triplet state carbenes have half-lives measured in seconds, and therefore can be observed but not stored.