3.-Electrochemical-Cells-V2-
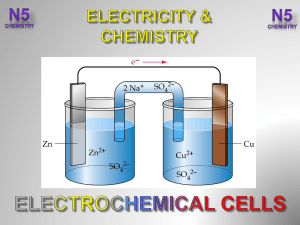
... (aq) decrease as they change into (s). The solution needs +ve ions to remain electrically neutral. ...
... (aq) decrease as they change into (s). The solution needs +ve ions to remain electrically neutral. ...
battery technology - EngineeringDuniya.com
... their discharge. There is no change in the electrolyte composition during the operation. It can be left unused for long periods of time at any state of charge without any appreciable damage (i.e. long shelf life). It can be encased as a sealed unit like the dry cell because gassing will not occur du ...
... their discharge. There is no change in the electrolyte composition during the operation. It can be left unused for long periods of time at any state of charge without any appreciable damage (i.e. long shelf life). It can be encased as a sealed unit like the dry cell because gassing will not occur du ...
Electricity Notes
... B. There are two kinds of basic circuits: series and parallel. 1. A series circuit has only one path for the electric current to follow—if the path is broken, the current will no longer flow and all devices in the circuit stop working. 2. A parallel circuit has more than one path for the electric c ...
... B. There are two kinds of basic circuits: series and parallel. 1. A series circuit has only one path for the electric current to follow—if the path is broken, the current will no longer flow and all devices in the circuit stop working. 2. A parallel circuit has more than one path for the electric c ...
MS PowerPoint - Catalysis Eprints database
... The electrified interface between the Colloidal particle and the medium causes a potential difference in the interface, which interacts with the externally applied electric field lies the basis for coating of metals. Is the friction between two solids in presence of liquid film an Electrified in ...
... The electrified interface between the Colloidal particle and the medium causes a potential difference in the interface, which interacts with the externally applied electric field lies the basis for coating of metals. Is the friction between two solids in presence of liquid film an Electrified in ...
AP* Chemistry ELECTROCHEMISTRY Terms to Know
... Nature has a way of returning metals to their natural states, which is often their ore. We call this process corrosion. It involves the oxidation of the metal, which causes it to lose its structural integrity and attractiveness. This is particularly troublesome when steel corrodes. The main componen ...
... Nature has a way of returning metals to their natural states, which is often their ore. We call this process corrosion. It involves the oxidation of the metal, which causes it to lose its structural integrity and attractiveness. This is particularly troublesome when steel corrodes. The main componen ...
Cells and Batteries File
... Reaction of a fuel, e.g., H2, with atmospheric O2, separated into half cells. Chemical energy is directly converted into electricity – high efficiency (40 – 60% but up to 85% in some cases). Catalysts are required at both anode and cathode. ◦ Anode: Pt, Pt/Ru mixtures (expensive) ◦ Cathode: Ni can b ...
... Reaction of a fuel, e.g., H2, with atmospheric O2, separated into half cells. Chemical energy is directly converted into electricity – high efficiency (40 – 60% but up to 85% in some cases). Catalysts are required at both anode and cathode. ◦ Anode: Pt, Pt/Ru mixtures (expensive) ◦ Cathode: Ni can b ...
Review Problems – Chapter 18 1. A large electrolytic cell that
... 1. A large electrolytic cell that produces metallic aluminum from Al2O3 ore is capable of making 250 kg of aluminum in 24 hours. Determine the current (in amps) that is required for this process. Include appropriate chemical reactions. [3.10 x 104 amps] ...
... 1. A large electrolytic cell that produces metallic aluminum from Al2O3 ore is capable of making 250 kg of aluminum in 24 hours. Determine the current (in amps) that is required for this process. Include appropriate chemical reactions. [3.10 x 104 amps] ...
Artificial Photosynthesis: A Workshop in Solar Cell Design
... substances, i.e. fuels, are produced in these reactions from very simple, low‐energy starting materials powered by a readily available and abundant form of energy in sunlight. Solar cells mimic the light absorption and electron flow of photosynthesis to produce electricity. The basic ...
... substances, i.e. fuels, are produced in these reactions from very simple, low‐energy starting materials powered by a readily available and abundant form of energy in sunlight. Solar cells mimic the light absorption and electron flow of photosynthesis to produce electricity. The basic ...
Answers for Review Questions Exam 3
... Secondary Battery – Uses a redox reaction, which is recharged by applying an external current. Ex. Lead storage battery. Ni-cad batteries, Problems: Lead Storage – High mass to power ratio, high mass but little power. 9. Electrolysis is the use of an electric current to bring about a chemical change ...
... Secondary Battery – Uses a redox reaction, which is recharged by applying an external current. Ex. Lead storage battery. Ni-cad batteries, Problems: Lead Storage – High mass to power ratio, high mass but little power. 9. Electrolysis is the use of an electric current to bring about a chemical change ...
Answers for Review Questions Exam 3
... 14. Because not all metals are easily oxidized by water. H2O and O2 are the two main substances. Keep the reactants apart: form a protective layer (ex. Oxide or Paint). Force the anode to act as a cathode: Use a zinc covering as a sacrificial anode. 15. Use a voltage of 4.6 V. This give you 1.0287 x ...
... 14. Because not all metals are easily oxidized by water. H2O and O2 are the two main substances. Keep the reactants apart: form a protective layer (ex. Oxide or Paint). Force the anode to act as a cathode: Use a zinc covering as a sacrificial anode. 15. Use a voltage of 4.6 V. This give you 1.0287 x ...
SNC1D Exam Review
... 8. For each of the circuits in #5 c) & d), assume that the power source supplies 6VDC and the resistors have resistances of 2 , 4 , and 6. Calculate: a) The current flowing through each resistor. b) The current flowing from the battery. c) The potential difference across each resistor. 9. Solve t ...
... 8. For each of the circuits in #5 c) & d), assume that the power source supplies 6VDC and the resistors have resistances of 2 , 4 , and 6. Calculate: a) The current flowing through each resistor. b) The current flowing from the battery. c) The potential difference across each resistor. 9. Solve t ...
Theory of solar cells

The theory of solar cells explains the physical and chemical processes by which photons are converted into electric current when striking a suitable semiconductor device. The theoretical studies are of practical use because they predict the fundamental limits of solar cell, and give guidance on the phenomena that contribute to losses and solar cell efficiency.