284
... conditions? 30. The more reactive halogen elements are able to replace the less reactive halogens from their compounds. For ex ample, if chlorine gas is bubbled through a potassium iodide solution, elemental iodine is produced. C12(g) + KI(aq) I2(s) + KCl(aq) Calculate the mass of iodine produced ...
... conditions? 30. The more reactive halogen elements are able to replace the less reactive halogens from their compounds. For ex ample, if chlorine gas is bubbled through a potassium iodide solution, elemental iodine is produced. C12(g) + KI(aq) I2(s) + KCl(aq) Calculate the mass of iodine produced ...
chemistry 11 exam review
... 6. 45 mL of gas at 15C and 790 torr is changed to 23C and 810 torr. What is the new volume? (45 mL) 7. 175 mL of gas at –30.0C and 2.57 atm is changed to standard conditions. What is the new volume? (505 mL) 8. What pressure is needed to change 130 mL of gas at 740 torr to 150 mL? (641 torr) 9. W ...
... 6. 45 mL of gas at 15C and 790 torr is changed to 23C and 810 torr. What is the new volume? (45 mL) 7. 175 mL of gas at –30.0C and 2.57 atm is changed to standard conditions. What is the new volume? (505 mL) 8. What pressure is needed to change 130 mL of gas at 740 torr to 150 mL? (641 torr) 9. W ...
How to balance chemical equations File
... see that they’re the same. A law in chemistry, the Law of Conservation of Mass, states, “In an ordinary chemical reaction, matter is neither created nor destroyed.” This means that you have neither gained nor lost any atoms during the reaction. They may be combined differently, but they’re still the ...
... see that they’re the same. A law in chemistry, the Law of Conservation of Mass, states, “In an ordinary chemical reaction, matter is neither created nor destroyed.” This means that you have neither gained nor lost any atoms during the reaction. They may be combined differently, but they’re still the ...
Lab Stuff - WW-P K
... 1. Carbon atoms make 4 bonds, and can arrange these bonds by using single, double, or triple bonds to other atoms. 2. Electron-dot and structural formulas describe the bonds between atoms in alkanes, alkenes and alkynes. 3. Petroleum is a nonrenewable resource, and many everyday materials are produc ...
... 1. Carbon atoms make 4 bonds, and can arrange these bonds by using single, double, or triple bonds to other atoms. 2. Electron-dot and structural formulas describe the bonds between atoms in alkanes, alkenes and alkynes. 3. Petroleum is a nonrenewable resource, and many everyday materials are produc ...
EFFECT OF LEWIS ACID IN TiCl4/MgCl2/THF/AlCl3 CATALYST
... The total element content in catalysts such as Ti, Mg, Ca, Fe, Zn and Al upon various mixed metal chlorides is listed in Table 2. The external surface compositions of all catalyst also were approximated by EDX technique, as shown in Table 2. The results showed that None-Al exhibited the highest of T ...
... The total element content in catalysts such as Ti, Mg, Ca, Fe, Zn and Al upon various mixed metal chlorides is listed in Table 2. The external surface compositions of all catalyst also were approximated by EDX technique, as shown in Table 2. The results showed that None-Al exhibited the highest of T ...
Unit 7 Packet
... Hydrazine (N2H4) and hydrogen peroxide are used together as rocket fuel. The products are nitrogen gas and water. ...
... Hydrazine (N2H4) and hydrogen peroxide are used together as rocket fuel. The products are nitrogen gas and water. ...
Dr. Baxley`s Thermodynamics Worksheet
... atoms is exothermic and is accompanied by an entropy decrease, explain why all chemical compounds decompose into individual atoms if heated to a high enough temperature. ...
... atoms is exothermic and is accompanied by an entropy decrease, explain why all chemical compounds decompose into individual atoms if heated to a high enough temperature. ...
Lab Stuff:
... 1. Carbon atoms make 4 bonds, and can arrange these bonds by using single, double, or triple bonds to other atoms. 2. Electron-dot and structural formulas describe the bonds between atoms in alkanes, alkenes and alkynes. 3. Petroleum is a nonrenewable resource, and many everyday materials are produc ...
... 1. Carbon atoms make 4 bonds, and can arrange these bonds by using single, double, or triple bonds to other atoms. 2. Electron-dot and structural formulas describe the bonds between atoms in alkanes, alkenes and alkynes. 3. Petroleum is a nonrenewable resource, and many everyday materials are produc ...
Unit 2
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
Unit 2
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
Lab Stuff
... 1. Carbon atoms make 4 bonds, and can arrange these bonds by using single, double, or triple bonds to other atoms. 2. Electron-dot and structural formulas describe the bonds between atoms in alkanes, alkenes and alkynes. 3. Petroleum is a nonrenewable resource, and many everyday materials are produc ...
... 1. Carbon atoms make 4 bonds, and can arrange these bonds by using single, double, or triple bonds to other atoms. 2. Electron-dot and structural formulas describe the bonds between atoms in alkanes, alkenes and alkynes. 3. Petroleum is a nonrenewable resource, and many everyday materials are produc ...
Slide 1 of 24
... Hindenburg erupted into a fireball. Within a short time, 210,000 cubic meters of hydrogen had burned and the airship was destroyed. The chemical reaction that occurred is “hydrogen combines with oxygen to produce water.” You will learn to represent this chemical reaction by a chemical equation. Slid ...
... Hindenburg erupted into a fireball. Within a short time, 210,000 cubic meters of hydrogen had burned and the airship was destroyed. The chemical reaction that occurred is “hydrogen combines with oxygen to produce water.” You will learn to represent this chemical reaction by a chemical equation. Slid ...
Types of Chemical Reactions
... Thousands of known chemical reactions occur in various systems. Memorizing the equations for so many chemical reactions would be difficult. It is more useful and realistic to classify reactions according to various similarities and regularities. ...
... Thousands of known chemical reactions occur in various systems. Memorizing the equations for so many chemical reactions would be difficult. It is more useful and realistic to classify reactions according to various similarities and regularities. ...
ACS Practice Test 1
... 41. Which is not a characteristic of ionic substances? (A) Their reactions are generally extremely slow. (B) They conduct an electric current when fused. (C) Those having a common ion exhibit some similar chemical properties. (D) They lower the vapor pressure of water when dissolved in it. (E) They ...
... 41. Which is not a characteristic of ionic substances? (A) Their reactions are generally extremely slow. (B) They conduct an electric current when fused. (C) Those having a common ion exhibit some similar chemical properties. (D) They lower the vapor pressure of water when dissolved in it. (E) They ...
Name - cloudfront.net
... If heat is released by a chemical system, an equal amount of heat will be ____. By what quantity must the heat capacity (J/oC) of an object be divided to obtain the specific heat (J/goC) of that material? 64. When energy is changed from one form to another, ____. 65. What happens to the energy produ ...
... If heat is released by a chemical system, an equal amount of heat will be ____. By what quantity must the heat capacity (J/oC) of an object be divided to obtain the specific heat (J/goC) of that material? 64. When energy is changed from one form to another, ____. 65. What happens to the energy produ ...
Chapter 11 Chemical Reactions
... Conservation of Mass) only balance by changing coefficients special symbols to indicate physical state, catalyst or energy required, etc. ...
... Conservation of Mass) only balance by changing coefficients special symbols to indicate physical state, catalyst or energy required, etc. ...
Chemistry 2008 Multiple Choice
... 32. Gaseous cyclobutene undergoes a first-order reaction to form gaseous butadiene. At a particular temperature, the partial pressure of cyclobutene in the reaction vessel drops to one-eighth its original value in 120 seconds. What is the half-life for this reaction at this temperature? (A) 15 s (B) ...
... 32. Gaseous cyclobutene undergoes a first-order reaction to form gaseous butadiene. At a particular temperature, the partial pressure of cyclobutene in the reaction vessel drops to one-eighth its original value in 120 seconds. What is the half-life for this reaction at this temperature? (A) 15 s (B) ...
fo-Balancing Chemical Notes
... Balancing Chemical Equations Chemical Reactions The following diagrams graphically illustrate two simple chemical reactions. Notice the difference between the subscripts (ex. H2) and the coefficients (shown in red) in these reactions. When balancing reactions, the coefficients can (and usually need ...
... Balancing Chemical Equations Chemical Reactions The following diagrams graphically illustrate two simple chemical reactions. Notice the difference between the subscripts (ex. H2) and the coefficients (shown in red) in these reactions. When balancing reactions, the coefficients can (and usually need ...
Document
... area involves the efficient, and highly enantioselective monofluoralkylation of alcohols using the Mitsunobu reaction ...
... area involves the efficient, and highly enantioselective monofluoralkylation of alcohols using the Mitsunobu reaction ...
Chemistry 2nd Semester Final Review
... Most things are mixtures There are only two types of pure substances: elements and compounds A mixture is anything that is not a single element or a single compound, but a mixture of more than one element or compound Mixtures can be separated through physical means. For example, if you want to remov ...
... Most things are mixtures There are only two types of pure substances: elements and compounds A mixture is anything that is not a single element or a single compound, but a mixture of more than one element or compound Mixtures can be separated through physical means. For example, if you want to remov ...
Name - Deans Community High School
... b) Is the forward reaction is exothermic or endothermic. ............................................ 1 c) Gold and platinum both catalyse the reaction. For the forward reaction EA using gold is 30 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the grap ...
... b) Is the forward reaction is exothermic or endothermic. ............................................ 1 c) Gold and platinum both catalyse the reaction. For the forward reaction EA using gold is 30 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the grap ...
Catalysis for a Sustainable World
... Newcastle upon Tyne. Professor North has published over 130 original papers and also holds six patents. His research interests are centred on the design and mechanistic study of new catalysts with applications including asymmetric carbon-carbon bond formation, carbon dioxide chemistry and polymer ch ...
... Newcastle upon Tyne. Professor North has published over 130 original papers and also holds six patents. His research interests are centred on the design and mechanistic study of new catalysts with applications including asymmetric carbon-carbon bond formation, carbon dioxide chemistry and polymer ch ...
Ch 11 Chemical Reactions
... Count the atoms of each type appearing on both sides Treat polyatomic ions like an “element” if they are unchanged by the reaction Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the pure elements until LAST! (things like H2, O2, ...
... Count the atoms of each type appearing on both sides Treat polyatomic ions like an “element” if they are unchanged by the reaction Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the pure elements until LAST! (things like H2, O2, ...
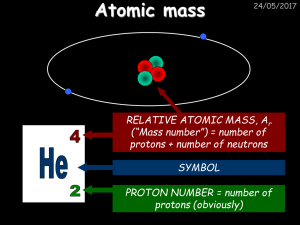
Atomic mass - drseemaljelani
... calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
... calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
Exam 3 - Canvas by Instructure
... Unknown A: _________________ Unknown B: _________________ Hint: There is more than one correct answer that is consistent with the data provided. D. Based on your from part C above, which gas would have the larger a & b terms in the van der Waals equation? Explain your reasoning by describing the dif ...
... Unknown A: _________________ Unknown B: _________________ Hint: There is more than one correct answer that is consistent with the data provided. D. Based on your from part C above, which gas would have the larger a & b terms in the van der Waals equation? Explain your reasoning by describing the dif ...
Catalytic reforming

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.In addition to a gasoline blending stock, reformate is the main source of aromatic bulk chemicals such as benzene, toluene, xylene and ethylbenzene which have diverse uses, most importantly as raw materials for conversion into plastics. However, the benzene content of reformate makes it carcinogenic, which has led to governmental regulations effectively requiring further processing to reduce its benzene content.This process is quite different from and not to be confused with the catalytic steam reforming process used industrially to produce products such as hydrogen, ammonia, and methanol from natural gas, naphtha or other petroleum-derived feedstocks. Nor is this process to be confused with various other catalytic reforming processes that use methanol or biomass-derived feedstocks to produce hydrogen for fuel cells or other uses.