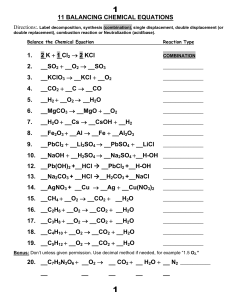
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... Which above reaction in #2 is a decomposition reaction? ______________________ Which above reaction is a single replacement reaction? ________________ Which above reaction is a combustion reaction? ______________ ...
... Which above reaction in #2 is a decomposition reaction? ______________________ Which above reaction is a single replacement reaction? ________________ Which above reaction is a combustion reaction? ______________ ...
Effect Of Convection For Gaseous Hydrochloride
... metals. It is collected as a dust. For the steel production, 6 millions ton/year in the Czech, 100 000 t of the dust /year is produced. It cannot be recycled as a iron feedstock because it contains not only 50% of Fe but also 5-15% Zn, mostly in form of zinc ferrite (franklinite) ZnFe2O4, and furthe ...
... metals. It is collected as a dust. For the steel production, 6 millions ton/year in the Czech, 100 000 t of the dust /year is produced. It cannot be recycled as a iron feedstock because it contains not only 50% of Fe but also 5-15% Zn, mostly in form of zinc ferrite (franklinite) ZnFe2O4, and furthe ...
GCSE_C2_Revision_+_Exam_Questions
... Substances that consist of simple molecules have only weak forces between the molecules (intermolecular forces). It is these intermolecular forces that are overcome, not the covalent bonds, when the substance melts or boils. Substances that consist of simple molecules do not conduct electricity beca ...
... Substances that consist of simple molecules have only weak forces between the molecules (intermolecular forces). It is these intermolecular forces that are overcome, not the covalent bonds, when the substance melts or boils. Substances that consist of simple molecules do not conduct electricity beca ...
Chemical Reactions and Equations
... (b) Rancidity- The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such a ...
... (b) Rancidity- The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such a ...
Chemical Reactions and Equations
... (b) Rancidity- The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such a ...
... (b) Rancidity- The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such a ...
Biodiesel preparation in batch emulgation reactor
... nearly all countries (therefore these countries are less dependent on crude-oil imports). Biodiesel does not contain polycyclic aromatic hydrocarbons, sulfur compounds and halogenides [1]. Biodiesel contains about 10 % of oxygen which supports burning, thus the emission of ash and smokiness are less ...
... nearly all countries (therefore these countries are less dependent on crude-oil imports). Biodiesel does not contain polycyclic aromatic hydrocarbons, sulfur compounds and halogenides [1]. Biodiesel contains about 10 % of oxygen which supports burning, thus the emission of ash and smokiness are less ...
Reversible and irreversible reactions - Chemwiki
... In this case also some amount of gaseous hydrogen iodide will be left unreacted. This means that the products of certain reactions can be converted back to the reactants. These types of reactions are called reversible reactions. Thus, in reversible reactions the products can react with one another u ...
... In this case also some amount of gaseous hydrogen iodide will be left unreacted. This means that the products of certain reactions can be converted back to the reactants. These types of reactions are called reversible reactions. Thus, in reversible reactions the products can react with one another u ...
What are the general types of reactions?
... – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after ...
... – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after ...
Chemistry 1: Second Semester Practice Exam Read each question
... 24. Given the reaction: 2KClO3 Æ 2 KCl + 3O2, What is the total number of moles of KCl produced when 1.50 moles of potassium chlorate is decomposed? C. 3.00 A. 1.50 B. 4.50 D. 0.750 25. Given the reaction: N2 (g) + 3 H2 (g) Æ 2 NH3 (g). How many liters of ammonia measured at STP are produced when 2 ...
... 24. Given the reaction: 2KClO3 Æ 2 KCl + 3O2, What is the total number of moles of KCl produced when 1.50 moles of potassium chlorate is decomposed? C. 3.00 A. 1.50 B. 4.50 D. 0.750 25. Given the reaction: N2 (g) + 3 H2 (g) Æ 2 NH3 (g). How many liters of ammonia measured at STP are produced when 2 ...
Biochemistry I (CHE 418 / 5418)
... If 2.10 mol N2 and 5.70 mol H2 react, what is the limiting reagent. Ask the question, how many mol of H2 is needed to react with .10 ...
... If 2.10 mol N2 and 5.70 mol H2 react, what is the limiting reagent. Ask the question, how many mol of H2 is needed to react with .10 ...
MCQ plus answers
... The following multiple choice questions are provided to illustrate the type of questions used in this section of the paper and to provide you with extra practice. It is not a sample quiz. The questions in the paper will be in the style of these questions but may well cover different topics. In the e ...
... The following multiple choice questions are provided to illustrate the type of questions used in this section of the paper and to provide you with extra practice. It is not a sample quiz. The questions in the paper will be in the style of these questions but may well cover different topics. In the e ...
C:\Users\mrh70950\Documents\My Files\WordPerfect
... i. anti-addition stereochemistry yields 1-alkenes from terminal alkynes and (E)-alkenes from internal alkynes 2. double hydrogenation: addition of 2 mol of H2 to yield alkanes a. noble metal catalyst + excess H2 3. electrophilic additions (all by very similar mechanisms) a. hydrohalogenation: addit ...
... i. anti-addition stereochemistry yields 1-alkenes from terminal alkynes and (E)-alkenes from internal alkynes 2. double hydrogenation: addition of 2 mol of H2 to yield alkanes a. noble metal catalyst + excess H2 3. electrophilic additions (all by very similar mechanisms) a. hydrohalogenation: addit ...
solutions - UMass Chemistry
... 13. (10 pts) Insoluble Mg(OH)2 is placed into a solution of nitric acid, HNO3. Write the net ionic equation that occurs. If no reaction occurs, say so. This will be an acid-base reaction, so H2O is a product. Nitric acid is a strong acid, meaning that it will start out completely ionized into H+ (aq ...
... 13. (10 pts) Insoluble Mg(OH)2 is placed into a solution of nitric acid, HNO3. Write the net ionic equation that occurs. If no reaction occurs, say so. This will be an acid-base reaction, so H2O is a product. Nitric acid is a strong acid, meaning that it will start out completely ionized into H+ (aq ...
7 - Mona Shores Blogs
... 69. Which of the following numbers has three significant figures? a. 1.00 b. .00345 c. 678,000 d. they all do 70. Which of the following is not a necessary component of a neutral atom? a. One or more electrons b. One or more protons c. One or more neutrons d. A nucleus 71. Which of the following is ...
... 69. Which of the following numbers has three significant figures? a. 1.00 b. .00345 c. 678,000 d. they all do 70. Which of the following is not a necessary component of a neutral atom? a. One or more electrons b. One or more protons c. One or more neutrons d. A nucleus 71. Which of the following is ...
"Introduction" Kinetics in Process Chemistry: Case Studies Baran Group Meeting Mike DeMartino
... N,N'-carbonyldiimidazole is commonly used as an acid activator for coupling reactions. There are advantages to using CDI: price -$8/mol (large-scale purchase), and the byproducts are the innocuous CO2 and imidazole. It is not without its problems though. The acyl imidazole is less reactive than, for ...
... N,N'-carbonyldiimidazole is commonly used as an acid activator for coupling reactions. There are advantages to using CDI: price -$8/mol (large-scale purchase), and the byproducts are the innocuous CO2 and imidazole. It is not without its problems though. The acyl imidazole is less reactive than, for ...
Mole Equation Homework Hint: Start equations with the numbers
... 3. Iron (III) oxide is formed when iron combines with oxygen. How many grams of Fe2O3 are formed when 16.7 g of Fe reacts completely with oxygen? 4Fe(s) + 3O2(g) --> 2Fe2 O3(s) ...
... 3. Iron (III) oxide is formed when iron combines with oxygen. How many grams of Fe2O3 are formed when 16.7 g of Fe reacts completely with oxygen? 4Fe(s) + 3O2(g) --> 2Fe2 O3(s) ...
2014-15 FINAL REVIEW Nomenclature: Chemical Name Chemical
... 1. A child has a toy balloon with a volume of 1.80 liters. The temperature of the balloon when it was filled was 200 C and the pressure was 1.00 atm. If the child were to let go of the balloon and it rose 3 kilometers into the sky where the pressure is 0.667 atm and the temperature is -100 C, what w ...
... 1. A child has a toy balloon with a volume of 1.80 liters. The temperature of the balloon when it was filled was 200 C and the pressure was 1.00 atm. If the child were to let go of the balloon and it rose 3 kilometers into the sky where the pressure is 0.667 atm and the temperature is -100 C, what w ...
Document
... • only happens if one product: a) doesn’t dissolve in water & forms solid (a “precipitate”), or b) is gas that bubbles out, or c) is molecular compound (usually water) ...
... • only happens if one product: a) doesn’t dissolve in water & forms solid (a “precipitate”), or b) is gas that bubbles out, or c) is molecular compound (usually water) ...
Practice Exam #2 with Answers
... water bath at 99°C. The barometric pressure is 753 torr. If the mass of the liquid retained in the flask is 1.362 g, what is its molar mass? a. ...
... water bath at 99°C. The barometric pressure is 753 torr. If the mass of the liquid retained in the flask is 1.362 g, what is its molar mass? a. ...
File
... Further oxidation of Y to Z occurs in the atmosphere. In this further oxidation, 1 mol of Y reacts with 0.5 mol of gaseous oxygen molecules. X could be either nitrogen or sulfur. Which statements about X, Y and Z can be correct? ...
... Further oxidation of Y to Z occurs in the atmosphere. In this further oxidation, 1 mol of Y reacts with 0.5 mol of gaseous oxygen molecules. X could be either nitrogen or sulfur. Which statements about X, Y and Z can be correct? ...
Candidates should check the question paper to
... e) Write a full chemical equation of the reaction including the condition of temperature in the preparation of ethane. ...
... e) Write a full chemical equation of the reaction including the condition of temperature in the preparation of ethane. ...
Thermo Practice Test
... 26. T - F For the process in #25, we would expect S to decrease with increasing pressure. 27. T - F For the decomposition of water to the elements at standard conditions, G= +56.7 kcal. This means that at least 56.7 kcal of work (energy) has to be supplied to make this reaction go. ...
... 26. T - F For the process in #25, we would expect S to decrease with increasing pressure. 27. T - F For the decomposition of water to the elements at standard conditions, G= +56.7 kcal. This means that at least 56.7 kcal of work (energy) has to be supplied to make this reaction go. ...
New Advances in Catalytic Systems for Conversion of CH4 and CO2
... a wide range of investigations for photo-induced reactions has been undertaken in some famous laboratories, such as the Brookhaven National Laboratory, the Lawrence Berkeley National Laboratory, etc. In 1996, Margl et al. [8] also reported that the Rhphosphine complex can catalyze the carbonylation ...
... a wide range of investigations for photo-induced reactions has been undertaken in some famous laboratories, such as the Brookhaven National Laboratory, the Lawrence Berkeley National Laboratory, etc. In 1996, Margl et al. [8] also reported that the Rhphosphine complex can catalyze the carbonylation ...
2014 Academic Challenge Sectional Chemistry Exam Solution Set 1
... nature of the reaction requires EAfwd to be less than EArev. It is not required that this obey a first order rate law or be a gas phase reaction (B or D). The dissociation of chlorine gas into chlorine atoms would be an endothermic reaction due to the bond breaking (C). There are no intermediates in ...
... nature of the reaction requires EAfwd to be less than EArev. It is not required that this obey a first order rate law or be a gas phase reaction (B or D). The dissociation of chlorine gas into chlorine atoms would be an endothermic reaction due to the bond breaking (C). There are no intermediates in ...
Catalytic reforming

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.In addition to a gasoline blending stock, reformate is the main source of aromatic bulk chemicals such as benzene, toluene, xylene and ethylbenzene which have diverse uses, most importantly as raw materials for conversion into plastics. However, the benzene content of reformate makes it carcinogenic, which has led to governmental regulations effectively requiring further processing to reduce its benzene content.This process is quite different from and not to be confused with the catalytic steam reforming process used industrially to produce products such as hydrogen, ammonia, and methanol from natural gas, naphtha or other petroleum-derived feedstocks. Nor is this process to be confused with various other catalytic reforming processes that use methanol or biomass-derived feedstocks to produce hydrogen for fuel cells or other uses.























