Chemistry 143 Experiment #11 AcidBase Titration Dr. Caddell
... goggles at all times. All solutions may go down the drain. If you discard any 6M NaOH dilute it with tap water before pouring it down the drain. Introduction In part A of this experiment you will make a solution of sodium hydroxide, your stock solution, by diluting some approximately 6M sodium ...
... goggles at all times. All solutions may go down the drain. If you discard any 6M NaOH dilute it with tap water before pouring it down the drain. Introduction In part A of this experiment you will make a solution of sodium hydroxide, your stock solution, by diluting some approximately 6M sodium ...
UNIT I: Introduction to Chemistry
... Explain how some isotopes are made of unstable nuclei, which decay over time emitting particles and energy. b. Contrast the three kinds of emissions (alpha, beta, and gamma), the composition of the emission, and the material required to shield them. c. Differentiate between nuclear fission and fusio ...
... Explain how some isotopes are made of unstable nuclei, which decay over time emitting particles and energy. b. Contrast the three kinds of emissions (alpha, beta, and gamma), the composition of the emission, and the material required to shield them. c. Differentiate between nuclear fission and fusio ...
Chemical Equations - Salem Community Schools
... aqueous solution produce water or a gas (or both) rather than a precipitate. In such cases, the water or gas is shown as a product in the net ionic equation, as are the ions that produced it. The remaining ions are eliminated as spectator ions. The following example problem illustrates this concept. ...
... aqueous solution produce water or a gas (or both) rather than a precipitate. In such cases, the water or gas is shown as a product in the net ionic equation, as are the ions that produced it. The remaining ions are eliminated as spectator ions. The following example problem illustrates this concept. ...
12 U Chem Review
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
sch4ureview
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
Pdf - Text of NPTEL IIT Video Lectures
... is constant that means del U del v at s dv. We know the property relation du is Tds minus pdv. For a pure substance, the property relation du is Tds minus pdv. If you compare this equation with this one, or the first two terms of this, The first two terms are similar to this term as for a single com ...
... is constant that means del U del v at s dv. We know the property relation du is Tds minus pdv. For a pure substance, the property relation du is Tds minus pdv. If you compare this equation with this one, or the first two terms of this, The first two terms are similar to this term as for a single com ...
Limiting - Faculty Web Pages
... Check to see that the paper is free of creases and completely seals the holes in the funnel to make sure no solution will leak around the edges of the filter paper. With the vacuum turned on the solution to be filtered is poured into the Buchner funnel. Scrape out the container with a spatula and us ...
... Check to see that the paper is free of creases and completely seals the holes in the funnel to make sure no solution will leak around the edges of the filter paper. With the vacuum turned on the solution to be filtered is poured into the Buchner funnel. Scrape out the container with a spatula and us ...
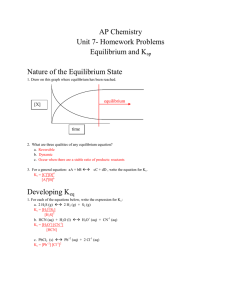
① Name AP CHEM __/__/__ Chapter 12 Outline
... If we choose to consider conditions where the reverse reaction can be neglected, the reaction rate will depend only on the concentrations of the reactants. Consider the following reaction: 2NO2(g) 2NO(g) + O2(g) For the above reaction the rate law is: Rate = k [NO2]n where k, the proportiona ...
... If we choose to consider conditions where the reverse reaction can be neglected, the reaction rate will depend only on the concentrations of the reactants. Consider the following reaction: 2NO2(g) 2NO(g) + O2(g) For the above reaction the rate law is: Rate = k [NO2]n where k, the proportiona ...
Part II - American Chemical Society
... score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 27, 2003, after which tests can be returned to students an ...
... score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 27, 2003, after which tests can be returned to students an ...
Volatility of Organic Aerosol: Evaporation of Ammonium Sulfate
... over a mixed particle surface are affected by the particle composition. This effect is described by the activity, i.e. the product of the activity coefficient and the molar fraction of the given compound in the particle. For aqueous solutions of single organic compounds directly measurement-based activi ...
... over a mixed particle surface are affected by the particle composition. This effect is described by the activity, i.e. the product of the activity coefficient and the molar fraction of the given compound in the particle. For aqueous solutions of single organic compounds directly measurement-based activi ...
HONORS LAB MANUAL - Tenafly High School
... large test tube. Heat the tube strongly over a laboratory burner for several minutes. Dispose in the solid waste container. 2. Mass a piece of copper wire. Record the mass. Place it in a small test tube and add silver nitrate until it is just covered. Place the test tube in a rack and let it stand u ...
... large test tube. Heat the tube strongly over a laboratory burner for several minutes. Dispose in the solid waste container. 2. Mass a piece of copper wire. Record the mass. Place it in a small test tube and add silver nitrate until it is just covered. Place the test tube in a rack and let it stand u ...
LECTURE PPT: Chapter 8
... • MTBE made its way into drinking water through gasoline spills at gas stations, from boat motors, and from leaking underground storage tanks. • Ethanol (C2H5OH), made from the fermentation of grains, is now used as a substitute for MTBE to increase oxygen content in motor fuel. • Ethanol was not us ...
... • MTBE made its way into drinking water through gasoline spills at gas stations, from boat motors, and from leaking underground storage tanks. • Ethanol (C2H5OH), made from the fermentation of grains, is now used as a substitute for MTBE to increase oxygen content in motor fuel. • Ethanol was not us ...
Principles of Chemistry 1 and 2 Notes
... {Consider only the central atoms which are the two carbon atom in this case]: (ground state): 2s2 2p2 -------> (excitation): 2s1 2p3 (excited state) -------> (hybridization) --------> sp2 + 2p Hybrid orbital sp is made of 1s orbital mixed with 2px1. Only 2px1 and 2pz1 are left unhyhridized. 2px1 and ...
... {Consider only the central atoms which are the two carbon atom in this case]: (ground state): 2s2 2p2 -------> (excitation): 2s1 2p3 (excited state) -------> (hybridization) --------> sp2 + 2p Hybrid orbital sp is made of 1s orbital mixed with 2px1. Only 2px1 and 2pz1 are left unhyhridized. 2px1 and ...
Introduction Statistical Thermodynamics
... Our basic assumption must be seriously wrong! ... but are we doing the statistics correctly? Monday, January 5, 15 ...
... Our basic assumption must be seriously wrong! ... but are we doing the statistics correctly? Monday, January 5, 15 ...
13738_2016_853_MOESM1_ESM
... procedure described above. The results in Fig. 2S indicate that ER increases from 3 to 5 mL and then remains constant. Therefore, 5 mL was selected as the optimum volume for the extractant. ...
... procedure described above. The results in Fig. 2S indicate that ER increases from 3 to 5 mL and then remains constant. Therefore, 5 mL was selected as the optimum volume for the extractant. ...
Phase behavior of clathrate hydrates: a model for single and
... where the molar volume of ice and liquid water were 9t to experimental data from Perry’s Handbook (Green & Maloney, 1997) and NIST (Chase Jr., 1998), respectively. The vapor pressures of the liquid water and ice are modeled using Eq. (9) and 9t to data from the CRC Handbook (Lide & Frederiskse, 1995 ...
... where the molar volume of ice and liquid water were 9t to experimental data from Perry’s Handbook (Green & Maloney, 1997) and NIST (Chase Jr., 1998), respectively. The vapor pressures of the liquid water and ice are modeled using Eq. (9) and 9t to data from the CRC Handbook (Lide & Frederiskse, 1995 ...
1st-Year-ch-wise-test
... (2) The filtration through filter paper is increased by using (a) sintered glass crucible (b) funnel of small stem (b) flutted filter paper (d) filter paper of greater porosity (3) A solvent chosen for crystallization should (a) not react chemically (b) react chemically (c) be expensive (d) dissolve ...
... (2) The filtration through filter paper is increased by using (a) sintered glass crucible (b) funnel of small stem (b) flutted filter paper (d) filter paper of greater porosity (3) A solvent chosen for crystallization should (a) not react chemically (b) react chemically (c) be expensive (d) dissolve ...
Assessment of feldspar solubility constants in water in the range of O
... different reaction schemes. Hemingway and Haselton (1994) recommended a value of ⫺3,935,000 J mol⫺1 for the enthalpy of formation of low-albite from the elements. It is valid for the natural low-albites from Amelia and Varuträsk. Hemingway and Haselton (1994) argued that the value of Navrotsky and ...
... different reaction schemes. Hemingway and Haselton (1994) recommended a value of ⫺3,935,000 J mol⫺1 for the enthalpy of formation of low-albite from the elements. It is valid for the natural low-albites from Amelia and Varuträsk. Hemingway and Haselton (1994) argued that the value of Navrotsky and ...