EXPERIMENT 10 Volumetric Analysis I Standardization of NaOH
... Introduction Titration is a common method of quantitative analysis used to determine the concentration of an unknown substance in a solution. The method is easy to use if the quantitative relationship between two reacting solutions is known. It is particularly well-suited to acid-base and oxidation- ...
... Introduction Titration is a common method of quantitative analysis used to determine the concentration of an unknown substance in a solution. The method is easy to use if the quantitative relationship between two reacting solutions is known. It is particularly well-suited to acid-base and oxidation- ...
The structure of strontium-doped hydroxyapatite
... structural modifications of HA as well as on the incorporation of the metal. Detailed structural characterization has been made by X-ray diffraction (XRD) in samples prepared at high temperatures by diffusion in the solid state, 4 and more recently by wet synthesis. 7 Solid-state diffusion produces ...
... structural modifications of HA as well as on the incorporation of the metal. Detailed structural characterization has been made by X-ray diffraction (XRD) in samples prepared at high temperatures by diffusion in the solid state, 4 and more recently by wet synthesis. 7 Solid-state diffusion produces ...
10. Solution Guide to Supplementary Exercises
... A All reactions cease. B The reactions have gone to completion. C The rates of the forward and backward reactions are equal. D The amount of products equals the amount of reactants. ...
... A All reactions cease. B The reactions have gone to completion. C The rates of the forward and backward reactions are equal. D The amount of products equals the amount of reactants. ...
Simulations of Si and SiO2 Etching in SF6+O2 Plasma
... required for device isolation, formation of vertical capacitors in integrated circuits and waveguides in optoelectronics. It prevents voids during trench refilling and avoids breakdown of dielectric film covering the trench sidewalls. Round corners reduce the build-up of mechanical stress onto a sil ...
... required for device isolation, formation of vertical capacitors in integrated circuits and waveguides in optoelectronics. It prevents voids during trench refilling and avoids breakdown of dielectric film covering the trench sidewalls. Round corners reduce the build-up of mechanical stress onto a sil ...
Document
... + The reaction is both enthalpically favored (exothermic) and entropically favored. The reaction is enthalpically favored (exothermic) and entropically opposed. ...
... + The reaction is both enthalpically favored (exothermic) and entropically favored. The reaction is enthalpically favored (exothermic) and entropically opposed. ...
Class XI Physical Chemistry Short note
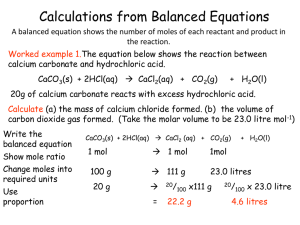
... Regardless of the amount of reactants added, the same products, with the same compositions, are formed (i.e. the precipitate observed in the reactions). However, if the reactants are not added in the correct ratios, there will be unreacted reactants that will remain in the final solution, together w ...
... Regardless of the amount of reactants added, the same products, with the same compositions, are formed (i.e. the precipitate observed in the reactions). However, if the reactants are not added in the correct ratios, there will be unreacted reactants that will remain in the final solution, together w ...
Graphical interpretation of analytical data from comparison of a field
... Westgard et al. [1–3] outlined the basic principles for method comparison in a clear, easy to follow manual. They also introduced the concept of allowable analytical error and gave an overview of published performance criteria. They recommended that the estimated analytical imprecision and bias be c ...
... Westgard et al. [1–3] outlined the basic principles for method comparison in a clear, easy to follow manual. They also introduced the concept of allowable analytical error and gave an overview of published performance criteria. They recommended that the estimated analytical imprecision and bias be c ...
2.0 Chem 20 Final Review
... • So why do we care about bonding capacity? – If we know how many bonding e-’s an atom has, we can predict what structure a molecular compound will have ...
... • So why do we care about bonding capacity? – If we know how many bonding e-’s an atom has, we can predict what structure a molecular compound will have ...
Appendix
... at the end point, the purity of the KHP, the molar mass for KHP, and the titration’s repeatability. Having established these, we can combine them to arrive at the final uncertainty. Uncertainty in the Mass of KHP. After drying the KHP, we store it in a sealed container to prevent it from readsorbing ...
... at the end point, the purity of the KHP, the molar mass for KHP, and the titration’s repeatability. Having established these, we can combine them to arrive at the final uncertainty. Uncertainty in the Mass of KHP. After drying the KHP, we store it in a sealed container to prevent it from readsorbing ...
Chemistry 180-213B Introductory Physical
... water under 1 standard atm (= 101325 Pa). We can measure T 0 by a gas thermometric method. At low pressures, the equation of state of a real gas can be written in the form pV = α + κ p. We measure the values of pV, α and κ at the above two fixed points.Considering that α is equal to nRT , we have T0 ...
... water under 1 standard atm (= 101325 Pa). We can measure T 0 by a gas thermometric method. At low pressures, the equation of state of a real gas can be written in the form pV = α + κ p. We measure the values of pV, α and κ at the above two fixed points.Considering that α is equal to nRT , we have T0 ...
Model for acid-base chemistry in nanoparticle growth (MABNAG)
... their condensational growth rates. However, all the vapours condensing on atmospheric nanoparticles and growing them to climatically relevant sizes are not identified yet and the effects of particle phase processes on particle growth rates are poorly known. Besides sulfuric acid, organic compounds a ...
... their condensational growth rates. However, all the vapours condensing on atmospheric nanoparticles and growing them to climatically relevant sizes are not identified yet and the effects of particle phase processes on particle growth rates are poorly known. Besides sulfuric acid, organic compounds a ...
The aim of this exercise is to study the acid... prepare a buffer solution. General Sciences Sample
... 2. Justify whether each of the following propositions is true or false: a) pH2 > pH1 b) pH1 = - log C1 c) pH2 = pH1 + 2 d) pH2 < 7 at 25°C. III- Reaction between Propanoic Acid and Sodium Hydroxide Solution The reaction between an aqueous solution of propanoic acid and an aqueous solution of sodium ...
... 2. Justify whether each of the following propositions is true or false: a) pH2 > pH1 b) pH1 = - log C1 c) pH2 = pH1 + 2 d) pH2 < 7 at 25°C. III- Reaction between Propanoic Acid and Sodium Hydroxide Solution The reaction between an aqueous solution of propanoic acid and an aqueous solution of sodium ...
Buffer Solutions
... of the anion of this salt, CHO2-1, to the acid, HCHO2, is needed? 3. A buffer solution was prepareed by dissolving 2.5 grams of NH4Cl in 125 mL of 0.24 M ammonia solution. At what pH will this solution serve as a buffer? 4. a) A formic acid, sodium formate solution is made up by dissolving 0.2 mole ...
... of the anion of this salt, CHO2-1, to the acid, HCHO2, is needed? 3. A buffer solution was prepareed by dissolving 2.5 grams of NH4Cl in 125 mL of 0.24 M ammonia solution. At what pH will this solution serve as a buffer? 4. a) A formic acid, sodium formate solution is made up by dissolving 0.2 mole ...
Acid Base Equilibrium Diploma Questions
... When KOH(aq) is added, the [HBb(aq)] increases and the solution turns yellow. When HCl(aq) ia added, the [H3O+(aq)] increases and the solution turns yellow. When HCl(aq) is added, the equilibrium shifts toward the reactants and the solution turns blue. When KOH(aq) is added, the equilibrium shifts t ...
... When KOH(aq) is added, the [HBb(aq)] increases and the solution turns yellow. When HCl(aq) ia added, the [H3O+(aq)] increases and the solution turns yellow. When HCl(aq) is added, the equilibrium shifts toward the reactants and the solution turns blue. When KOH(aq) is added, the equilibrium shifts t ...
Heterogeneous Electron-Transfer Kinetics for
... previously, and most importantly, the rate data at all bridge lengths studied here have not been previously21,28 determined over a range of temperatures. Both the oxidized and reduced forms of the ruthenium redox center are charged (+3 and +2, respectively) so that hydrophobic interactions with the ...
... previously, and most importantly, the rate data at all bridge lengths studied here have not been previously21,28 determined over a range of temperatures. Both the oxidized and reduced forms of the ruthenium redox center are charged (+3 and +2, respectively) so that hydrophobic interactions with the ...