Balancing Chemical Equations
... • The reactant chemical(s) are given on the left-hand side and the product chemical(s) on the right-hand side. ...
... • The reactant chemical(s) are given on the left-hand side and the product chemical(s) on the right-hand side. ...
Physical Science Chapter 6
... beginning substances (on the left) called reactants; ending substances (on the right) called products; arrow in the middle means “yields” or “gives”. ...
... beginning substances (on the left) called reactants; ending substances (on the right) called products; arrow in the middle means “yields” or “gives”. ...
Stoichiometry - WordPress.com
... • It is rare that we will have the exact amount of each reactant for a reaction. • One or more reactants are in excess – ie there is more than what is needed. • The reactant that is completely used up is said to be the limiting reactant. • It is the limiting reactant that must be used in stoichiomet ...
... • It is rare that we will have the exact amount of each reactant for a reaction. • One or more reactants are in excess – ie there is more than what is needed. • The reactant that is completely used up is said to be the limiting reactant. • It is the limiting reactant that must be used in stoichiomet ...
ap chemistry – 2013-2014
... Vapor pressure and changes in state Heating and cooling curves Composition of solutions Colloids and suspensions Separation techniques Effect on biological systems Unit 9: Kinetics Rates of reactions Factors that effect rates of reactions collision theory Reaction Pathways Rate equation determinatio ...
... Vapor pressure and changes in state Heating and cooling curves Composition of solutions Colloids and suspensions Separation techniques Effect on biological systems Unit 9: Kinetics Rates of reactions Factors that effect rates of reactions collision theory Reaction Pathways Rate equation determinatio ...
Amounts of Reactants and Products
... product that can be formed It is important to figure out which reactant is the limiting one so that you can correctly calculate the products that will be formed. Steps for Solving problems involving Limiting reactants: 1. Write and balance the equation for the reaction. 2. Convert known masses of ...
... product that can be formed It is important to figure out which reactant is the limiting one so that you can correctly calculate the products that will be formed. Steps for Solving problems involving Limiting reactants: 1. Write and balance the equation for the reaction. 2. Convert known masses of ...
CHEM 1212 Module Ten-Chapter 16 Name
... You may use assumptions I this calculation, show and prove assumption ...
... You may use assumptions I this calculation, show and prove assumption ...
Lab announcements – 2 lab quiz week before spring break
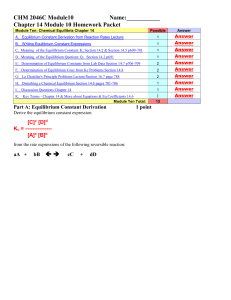
... simultaneously at the same rate ‘equilibrium’ doesn’t necessarily mean ‘equal’ amounts of reactants and products – in fact, it usually doesn’t. Equilibrium constant – measure of this balance aA + Kc ...
... simultaneously at the same rate ‘equilibrium’ doesn’t necessarily mean ‘equal’ amounts of reactants and products – in fact, it usually doesn’t. Equilibrium constant – measure of this balance aA + Kc ...
Unit 13 Worksheet Answers
... b. What happens to the amount of N 2 O 4 when the temperature is increased? decrease c. What happens to the amount of NO 2 when N 2 O 4 is added? Increase d. In what direction will the equilibrium shift when the volume of the container is decreased? right e. Is this reaction endothermic or exothermi ...
... b. What happens to the amount of N 2 O 4 when the temperature is increased? decrease c. What happens to the amount of NO 2 when N 2 O 4 is added? Increase d. In what direction will the equilibrium shift when the volume of the container is decreased? right e. Is this reaction endothermic or exothermi ...
one
... • Step 2 – change one or more coefficients until the equation is balanced. – Start by balancing an element that appears in only one reactant and product. – Once one element is balanced, proceed to balance another, and another, until all elements are balanced. – Balance chemical formulas by placing c ...
... • Step 2 – change one or more coefficients until the equation is balanced. – Start by balancing an element that appears in only one reactant and product. – Once one element is balanced, proceed to balance another, and another, until all elements are balanced. – Balance chemical formulas by placing c ...
2 Combustion in Gas
... (constant pressure and enthalpy), initial temperature (300 K) and pressure (1 atm) are entered on the Reactor Physical Properties tab. An estimated solution temperature of 2000 K is used to help ensure that the solution obtained is for an ignited gas rather than the unburned state. The presence of a ...
... (constant pressure and enthalpy), initial temperature (300 K) and pressure (1 atm) are entered on the Reactor Physical Properties tab. An estimated solution temperature of 2000 K is used to help ensure that the solution obtained is for an ignited gas rather than the unburned state. The presence of a ...
Chemical Equilibrium – Le Chatelier`s Principle
... of high ionic strength. In those circumstances the quotient of activity coefficients is effectively constant and the equilibrium constant is taken to be a concentration quotient. ...
... of high ionic strength. In those circumstances the quotient of activity coefficients is effectively constant and the equilibrium constant is taken to be a concentration quotient. ...
Chem 12 UNIT TWO CHEMICAL EQUILIBRIUM 7.1 REVERSIBLE
... A reaction with neg ΔH and pos S will always be favoured because it satisfies both conditions that drive a rxn to spontaneity. (A reaction with pos ΔH and neg S will NEVER BE SPONTANEOUS) - AN ENDOTHERMIC RXN will only occur if it has increasing entropy. ...
... A reaction with neg ΔH and pos S will always be favoured because it satisfies both conditions that drive a rxn to spontaneity. (A reaction with pos ΔH and neg S will NEVER BE SPONTANEOUS) - AN ENDOTHERMIC RXN will only occur if it has increasing entropy. ...
Chemistry Syllabus
... Data Table: The data table has a title that describes the information recorded in the table. It should contain neat (use a ruler) column and row headings. If a table is not provided, you will have to draw one. Units (ºC, mol, g, mL, etc.) must be recorded with each piece of data recorded in the da ...
... Data Table: The data table has a title that describes the information recorded in the table. It should contain neat (use a ruler) column and row headings. If a table is not provided, you will have to draw one. Units (ºC, mol, g, mL, etc.) must be recorded with each piece of data recorded in the da ...