(Lithium Fluoride-Poly-Vinyl Alcohol) Composites
... Optical properties of polymers constitute an important aspects in study of electronic transition and the possibility of their application as optical filters, a cover in solar collection, selection surfaces and green house. The information about the electronic structure of crystalline and amorphous s ...
... Optical properties of polymers constitute an important aspects in study of electronic transition and the possibility of their application as optical filters, a cover in solar collection, selection surfaces and green house. The information about the electronic structure of crystalline and amorphous s ...
Plasmons, polaritons What are plasmons and what are
... respond fast enough to screen it. Most metals and semiconductors are reflective in the visible range because their plasmon frequency is in the ultraviolet. Some metals, such as copper and gold, have electronic interband transitions in the visible range, whereby specific energies (colors) are absor ...
... respond fast enough to screen it. Most metals and semiconductors are reflective in the visible range because their plasmon frequency is in the ultraviolet. Some metals, such as copper and gold, have electronic interband transitions in the visible range, whereby specific energies (colors) are absor ...
Optical Sources
... Modes with gain above the cavity loss have the potential to lase Gain distribution depends on the spontaneous emission band Wavelength width of the individual longitudinal modes depends on the reflectivity of the end faces Wavelength separation of the modes D depends on the length of the cavity ...
... Modes with gain above the cavity loss have the potential to lase Gain distribution depends on the spontaneous emission band Wavelength width of the individual longitudinal modes depends on the reflectivity of the end faces Wavelength separation of the modes D depends on the length of the cavity ...
Spectrometry 1 R
... • As for energy: the light with the highest energy will be the one with the highest frequency - that will be the one with the smallest wavelength. • Light of each color has a different wavelength - blue light has a shorter wavelength than red light. Blue light therefore has a larger number of peaks ...
... • As for energy: the light with the highest energy will be the one with the highest frequency - that will be the one with the smallest wavelength. • Light of each color has a different wavelength - blue light has a shorter wavelength than red light. Blue light therefore has a larger number of peaks ...
The Photoelectric Effect
... the metal. For the least strongly bound electrons (the ones easiest to tear away from the metal) this amount of work is known as the "work function" and is labelled Wo. These electrons will leave with the greatest kinetic energy KEmax which is given by ...
... the metal. For the least strongly bound electrons (the ones easiest to tear away from the metal) this amount of work is known as the "work function" and is labelled Wo. These electrons will leave with the greatest kinetic energy KEmax which is given by ...
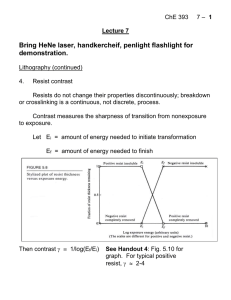
ChE 393 Course Notes
... well-defined molecule in the excited state that lives briefly before it decays into the largely repulsive ground state. Electronic diagram: ...
... well-defined molecule in the excited state that lives briefly before it decays into the largely repulsive ground state. Electronic diagram: ...
Atomic Emission Spectrometry - San Diego Unified School District
... heated or energized with electricity their electrons can gain energy. This promotes them to the higher-energy shell. When an electron is in a higher-energy shell it is said to be in an excited state. Electrons in excited states do not usually stay in them for very long. When electrons lose their ene ...
... heated or energized with electricity their electrons can gain energy. This promotes them to the higher-energy shell. When an electron is in a higher-energy shell it is said to be in an excited state. Electrons in excited states do not usually stay in them for very long. When electrons lose their ene ...
Wave Particle Unity and a Physically Realist Interpretation of Light
... wave and centered at the location of scattering. The second process involves light which is not scattered being 'pushed aside' (the Renninger (5) effect) creating a shadow in the given direction and increasing the density (and hence also the chances of absorption) from other locations. I will not de ...
... wave and centered at the location of scattering. The second process involves light which is not scattered being 'pushed aside' (the Renninger (5) effect) creating a shadow in the given direction and increasing the density (and hence also the chances of absorption) from other locations. I will not de ...
The Role of Ions in Body Chemistry Negative Ion Report: The CBS
... Each living cell appears to be a tiny battery generating its own current by chemical action, ie. the Sodium/Potassium Pump. When ions are in motion, they are invariably associated with magnetic fields; these fields have electro-magnetic qualities, therefore underlying the variations in these fields ...
... Each living cell appears to be a tiny battery generating its own current by chemical action, ie. the Sodium/Potassium Pump. When ions are in motion, they are invariably associated with magnetic fields; these fields have electro-magnetic qualities, therefore underlying the variations in these fields ...
On the use of ERL for gamma production
... power lasers are necessary. Now they are available near 1 micron wavelength. For This wavelength electron energy about 1.5 GeV is required. Modern accelerator technology may provide 1 A electron beam at maximum charge per bunch 5 nC and normalized emittance about 10 mmmrad. It corresponds to maximu ...
... power lasers are necessary. Now they are available near 1 micron wavelength. For This wavelength electron energy about 1.5 GeV is required. Modern accelerator technology may provide 1 A electron beam at maximum charge per bunch 5 nC and normalized emittance about 10 mmmrad. It corresponds to maximu ...
OQLECTURE14
... In the semi-classical model atoms are quantized, but light is not. as an additional part of the Hamiltonian. The evolution of the system is obtained by solving the Schrodinger equation with the total Hamiltonian. This is frequently impossible to solve analytically and we have to resort to approximat ...
... In the semi-classical model atoms are quantized, but light is not. as an additional part of the Hamiltonian. The evolution of the system is obtained by solving the Schrodinger equation with the total Hamiltonian. This is frequently impossible to solve analytically and we have to resort to approximat ...
Chapter 6. Light Source and Detectors
... Photoelectric Effect (2) • Classical theory said that E of ejected electron should increase with increase in light intensity — not observed! Experimental ...
... Photoelectric Effect (2) • Classical theory said that E of ejected electron should increase with increase in light intensity — not observed! Experimental ...
Jean Brainard, Ph.D.
... Like fluoride, other negative ions usually have names ending in –ide. Positive ions, on the other hand, are just given the element name followed by the word ion. For example, when a sodium atom loses an electron, it becomes a positive sodium ion. The charge of an ion is indicated by a plus (+) or mi ...
... Like fluoride, other negative ions usually have names ending in –ide. Positive ions, on the other hand, are just given the element name followed by the word ion. For example, when a sodium atom loses an electron, it becomes a positive sodium ion. The charge of an ion is indicated by a plus (+) or mi ...
Photonics. Fundamentals of Photonics and Physics. Volume 1. A Wiley- Brochure
... - Emphasizes processes and applications that specifically exploit photon attributes of light - Deals with the rapidly advancing area of modern optics - Chapters are written by top scientists in their field Written for the graduate level student in physical sciences; Industrial and academic researche ...
... - Emphasizes processes and applications that specifically exploit photon attributes of light - Deals with the rapidly advancing area of modern optics - Chapters are written by top scientists in their field Written for the graduate level student in physical sciences; Industrial and academic researche ...
General Properties of Light c = 3.0 x 108 m/s .
... pumping levels in long fibers, higher order Raman spectra can be generated by using the Raman spectrum as a new starting point, thereby building a chain of new spectra with decreasing amplitude. The disadvantage of intrinsic noise due to the initial spontaneous process can be overcome by seeding a s ...
... pumping levels in long fibers, higher order Raman spectra can be generated by using the Raman spectrum as a new starting point, thereby building a chain of new spectra with decreasing amplitude. The disadvantage of intrinsic noise due to the initial spontaneous process can be overcome by seeding a s ...
Soft X-ray spectroscopy of single sized CdS nanocrystals: size
... By linear extrapolation we determine the shift of the highenergy cut-off in the nanoparticle SXE spectra relative to the bulk case (DESXE in Table 1) which we take as a measure for the shift of the VB maximum (VBM) position [21]. 4 Adding the SXS shifts of VBM and CBM one obtains the total band gap ...
... By linear extrapolation we determine the shift of the highenergy cut-off in the nanoparticle SXE spectra relative to the bulk case (DESXE in Table 1) which we take as a measure for the shift of the VB maximum (VBM) position [21]. 4 Adding the SXS shifts of VBM and CBM one obtains the total band gap ...
11. Electro
... The photon will have a specific wavelength that depends on the energy difference between the excited state and the ground state. If the photon encounters another atom that has an electron in the same excited state, it can induce the electron to lose its energy in turn (called stimulated emission ...
... The photon will have a specific wavelength that depends on the energy difference between the excited state and the ground state. If the photon encounters another atom that has an electron in the same excited state, it can induce the electron to lose its energy in turn (called stimulated emission ...
Abstract
... on a generalized Boltzmann-type equation, including energy-drift and anti-diffusion effects, interband excitation, Coulomb scattering, thermal exchange with the lattice, etc. The kinetic Fokker-Planck equation describes the physics of these processes and can be used to explain how they lead to a las ...
... on a generalized Boltzmann-type equation, including energy-drift and anti-diffusion effects, interband excitation, Coulomb scattering, thermal exchange with the lattice, etc. The kinetic Fokker-Planck equation describes the physics of these processes and can be used to explain how they lead to a las ...
Mass Spectroscopy
... ionisation/fragmentography This is where gaseous effluent from usually a GC is bombarded with electrons from a filament Traditionally 70 eV used for ionisation and when most chemical bonds have 4- 7 eV of energy it is not surprising to see extensive fragmentation producing both +ve and –ve ions. ...
... ionisation/fragmentography This is where gaseous effluent from usually a GC is bombarded with electrons from a filament Traditionally 70 eV used for ionisation and when most chemical bonds have 4- 7 eV of energy it is not surprising to see extensive fragmentation producing both +ve and –ve ions. ...
EXAM IN COURSE TFY4220 Solid State Physics
... In total, with s atoms in basis, there are 3s modes, 3 acoustical and 3(s-1) optical modes. c) The free electron model can describe heat capacity, thermal conductivity, electrical conductivity/ resistivity and electrodynamics of metals. And the Hall effect (but not the signs in the Hall constant!). ...
... In total, with s atoms in basis, there are 3s modes, 3 acoustical and 3(s-1) optical modes. c) The free electron model can describe heat capacity, thermal conductivity, electrical conductivity/ resistivity and electrodynamics of metals. And the Hall effect (but not the signs in the Hall constant!). ...
... The combination explains free electrons with high absorption (R near zero) for low frequencies ( o 0 ) for the IR region of the light spectrum. The bound electrons, oscillator explain the absorption bands. Insulators and semiconductors are explained by the harmonic oscillator of bound electrons. ...