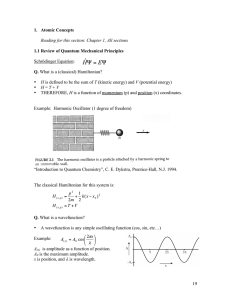
1.3 Atomic Concepts
... NOTE: Not all mathematical wavefunctions have the appropriate properties required by quantum mechanics! 1. Single-valued 2. First derivative w.r.t. position must be continuous 3. The wavefunction must vanish at any x where the potential is infinite. Q. What is an eigenvalue? ...
... NOTE: Not all mathematical wavefunctions have the appropriate properties required by quantum mechanics! 1. Single-valued 2. First derivative w.r.t. position must be continuous 3. The wavefunction must vanish at any x where the potential is infinite. Q. What is an eigenvalue? ...
A1993LX38200001
... at New York University in 1933-1934,1 had been fascinated by scattering theory. (When Richard Feynman was working on his dissertation, "The Principle of Least Action in Quantum Mechanics," in 1942, we liked to say, "Everything is scattering.") Since a second postdoctoral year with Niels Bohr in 1934 ...
... at New York University in 1933-1934,1 had been fascinated by scattering theory. (When Richard Feynman was working on his dissertation, "The Principle of Least Action in Quantum Mechanics," in 1942, we liked to say, "Everything is scattering.") Since a second postdoctoral year with Niels Bohr in 1934 ...
Document
... 1925 Heisenberg states uncertainty principle 1926 Schrodinger develops wave equation 1924-6 Boson and Fermion distributions developed ...
... 1925 Heisenberg states uncertainty principle 1926 Schrodinger develops wave equation 1924-6 Boson and Fermion distributions developed ...
Quantum Theory
... ___________ Electrons have ___________ energies Lower energy- ___________ to nucleus Higher energy- ___________ from nucleus Electrons can ___________ energy to raise to the next energy level and ___________ the same energy when falling to the ground state This model works well for ___________ but n ...
... ___________ Electrons have ___________ energies Lower energy- ___________ to nucleus Higher energy- ___________ from nucleus Electrons can ___________ energy to raise to the next energy level and ___________ the same energy when falling to the ground state This model works well for ___________ but n ...
Deriving E = mc /22 of Einstein`s ordinary quantum relativity energy
... where m is the mass, c is the speed of light and Ep is the esoterically large Planck energy thought to be in the region of 1019 Gev [3,4,6] [15-24]. In [20] Magueijo and Smolin combined quantum field theory, relativity and the idea of varying speed of light in a thoroughly ingenious way to produce a ...
... where m is the mass, c is the speed of light and Ep is the esoterically large Planck energy thought to be in the region of 1019 Gev [3,4,6] [15-24]. In [20] Magueijo and Smolin combined quantum field theory, relativity and the idea of varying speed of light in a thoroughly ingenious way to produce a ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 7. What is the nature of the path traced by a representative point in a two dimensional phase space for a one dimensional harmonic oscillator? 8. What is the nature of the new set of variables ( transformation from the set of variables ( , ) to ( , is zero? 9. What are coupled oscillators? ...
... 7. What is the nature of the path traced by a representative point in a two dimensional phase space for a one dimensional harmonic oscillator? 8. What is the nature of the new set of variables ( transformation from the set of variables ( , ) to ( , is zero? 9. What are coupled oscillators? ...
May 21, 2010 17:21 WSPC/INSTRUCTION FILE fortunato˙kazimieriz
... point, display behaviors qualitatively similar to those of the even core and they agree qualitatively with the model based on the E(5/4) boson-fermion symmetry. We describe then the U BF (5) to SU BF (3) transition when a fermion is allowed to occupy the orbits j = 1/2, 3/2, 5/2. The additional part ...
... point, display behaviors qualitatively similar to those of the even core and they agree qualitatively with the model based on the E(5/4) boson-fermion symmetry. We describe then the U BF (5) to SU BF (3) transition when a fermion is allowed to occupy the orbits j = 1/2, 3/2, 5/2. The additional part ...
On the Shoulders of Giants”
... William Rowan Hamilton’s publishes two papers on which it is possible to base all of mechanics and most of classical physics. Hamilton’s Principle is that a particle follows a path that minimizes L over a specific time interval (and consistent with any constraints). A constraint, for example, may be ...
... William Rowan Hamilton’s publishes two papers on which it is possible to base all of mechanics and most of classical physics. Hamilton’s Principle is that a particle follows a path that minimizes L over a specific time interval (and consistent with any constraints). A constraint, for example, may be ...
Neils Bohr
... • Bohr received a Nobel Prize in Physics for work of "for his services in the investigation of the structure of atoms and of the radiation emanating from them." • He continued to study new quantum principles which ultimately explained why light can be seen as a particle and wave but not at the same ...
... • Bohr received a Nobel Prize in Physics for work of "for his services in the investigation of the structure of atoms and of the radiation emanating from them." • He continued to study new quantum principles which ultimately explained why light can be seen as a particle and wave but not at the same ...
Notes27and29January2014BasicQuantumMechanics
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
QUANTUM CHAOS DOMINIQUE DELANDE Laboratoire Kastler-Brossel
... of memory of the initial conditions and ultimately for deterministic unpredictibility of the long time behaviour of the system. Most often, when the system is sensitive on initial conditions, it is also mixing and ergodic [1], i.e. a typical trajectory uniformly fills up the entire phase space at lo ...
... of memory of the initial conditions and ultimately for deterministic unpredictibility of the long time behaviour of the system. Most often, when the system is sensitive on initial conditions, it is also mixing and ergodic [1], i.e. a typical trajectory uniformly fills up the entire phase space at lo ...
Quantum Mechanics and General Relativity
... quarks, muons and other elementary particles. From this spring such applications as nuclear physics and solid state electronics. General Relativity on the other hand describes particularly the force of gravity and thus is usually applied to the largest and most massive structures and objects in the ...
... quarks, muons and other elementary particles. From this spring such applications as nuclear physics and solid state electronics. General Relativity on the other hand describes particularly the force of gravity and thus is usually applied to the largest and most massive structures and objects in the ...
SNC 1D0 – Chemistry Take Home Quiz
... 12. What are the rules for naming ionic compounds and molecular compounds? 13. Groups on the periodic table: a. where are the noble gases found on the periodic table: b. where are the alkali metals found on the periodic table: c. where are the halogens found on the periodic table: d. give and exampl ...
... 12. What are the rules for naming ionic compounds and molecular compounds? 13. Groups on the periodic table: a. where are the noble gases found on the periodic table: b. where are the alkali metals found on the periodic table: c. where are the halogens found on the periodic table: d. give and exampl ...
Quantum Complexity and Fundamental Physics
... QC’s Don’t Provide Exponential Speedups for Black-Box Search I.e., if you want more than the N Grover speedup for solving an NP-complete problem, then you’ll The “BBBV Noproblem SuperSearch Principle” can even need to exploit structure be applied in physicsBrassard, (e.g., to lower-bound [Bennett, ...
... QC’s Don’t Provide Exponential Speedups for Black-Box Search I.e., if you want more than the N Grover speedup for solving an NP-complete problem, then you’ll The “BBBV Noproblem SuperSearch Principle” can even need to exploit structure be applied in physicsBrassard, (e.g., to lower-bound [Bennett, ...
5.3_Matter_Waves
... has a probability wave - de Broglie Wavelength λ = h = Planck’s constant p momentum ...
... has a probability wave - de Broglie Wavelength λ = h = Planck’s constant p momentum ...
Erwin Schrodinger an Max Born and wavelength
... • an interpretation of quantum mechanics developed by Niels Bohr and his colleagues at the University of Copenhagen, based on the concept of wave-particle duality and the idea that the observation influences the results of an experiment ...
... • an interpretation of quantum mechanics developed by Niels Bohr and his colleagues at the University of Copenhagen, based on the concept of wave-particle duality and the idea that the observation influences the results of an experiment ...
Heisenberg`s uncertainty principle
... quantum mechanics shows that certain pairs of physical properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principl ...
... quantum mechanics shows that certain pairs of physical properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principl ...
Title of the course: Reaction Kinetics Credits: 3 Coordinator: Keszei
... simulations to calculate rate constants. Analytical and numerical solutions of large reaction mechanisms. Reduction of the number of species and reactions. Quasisteady-state approximation and its error. Reactions in liquid solutions. Ionic and polar transition states in polar solvents. Kinetic salt ...
... simulations to calculate rate constants. Analytical and numerical solutions of large reaction mechanisms. Reduction of the number of species and reactions. Quasisteady-state approximation and its error. Reactions in liquid solutions. Ionic and polar transition states in polar solvents. Kinetic salt ...
icnfp_2015_v5
... • Nontrivial interplay of gravity and quantum takes place not only at energies 1019 GeV, but also at normal Earthlike conditions. • The price to pay is extreme weakness. • We have seen a few examples in the history of physics then multiplicity saves the case (e.g. expected lifetime of the proton vs ...
... • Nontrivial interplay of gravity and quantum takes place not only at energies 1019 GeV, but also at normal Earthlike conditions. • The price to pay is extreme weakness. • We have seen a few examples in the history of physics then multiplicity saves the case (e.g. expected lifetime of the proton vs ...
Using Boolean Logic to Research Quantum Field Theory
... facts to explain QFT as a widely discussed subject in the field of science and mathematics itself. In comparison to other theories on the composition of out universe and matter itself, there is no conical definition for QFT. This has been quoted on the first paragraph of section one of the Stanford ...
... facts to explain QFT as a widely discussed subject in the field of science and mathematics itself. In comparison to other theories on the composition of out universe and matter itself, there is no conical definition for QFT. This has been quoted on the first paragraph of section one of the Stanford ...
CLASSICAL MECHANICS II - Makerere University Courses
... The wave equations; waves on strings; particles; waves in fluids; the general wave quation; solution of the wave equation; boundary conditions; Fourier series; waves in a rectangular box. Superposition and Interference of Waves Wave packets; phase and group velocities; de Broglie waves; energy densi ...
... The wave equations; waves on strings; particles; waves in fluids; the general wave quation; solution of the wave equation; boundary conditions; Fourier series; waves in a rectangular box. Superposition and Interference of Waves Wave packets; phase and group velocities; de Broglie waves; energy densi ...
Chapter 9 review
... If molecules, atoms, or subatomic particles are in the liquid or solid state, the Pauli exclusion principle prevents two particles with identical wave functions from sharing the same space. ...
... If molecules, atoms, or subatomic particles are in the liquid or solid state, the Pauli exclusion principle prevents two particles with identical wave functions from sharing the same space. ...