Chapter 1 INTRODUCTION AND BASIC CONCEPTS
... provides the necessary means to determine the quality as well as the degree of degradation of energy during a process. 3. The second law of thermodynamics is also used in determining the theoretical limits for the performance of commonly used engineering systems, such as heat engines and refrigerato ...
... provides the necessary means to determine the quality as well as the degree of degradation of energy during a process. 3. The second law of thermodynamics is also used in determining the theoretical limits for the performance of commonly used engineering systems, such as heat engines and refrigerato ...
Ezio Fornero, Kinetic Theory
... Kinetic theory is based on a set of hypotheses on the microscopic structure of matter founded on empirical observations. Some hypotheses apply to matter in general (also to liquid and solid phases), the others only to perfect gases. 1. Matter is composed of minimal units (“molecules”) which can be c ...
... Kinetic theory is based on a set of hypotheses on the microscopic structure of matter founded on empirical observations. Some hypotheses apply to matter in general (also to liquid and solid phases), the others only to perfect gases. 1. Matter is composed of minimal units (“molecules”) which can be c ...
國立台北科技大學 冷凍與空調工程研究所碩士在職專班
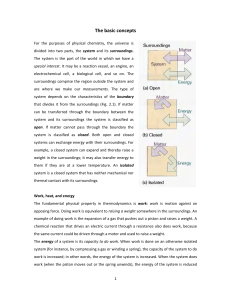
... as: heat flow, heat addition, heat rejection, heat removal , heat gain, heat loss, heat storage, heat generation, electrical heating, resistance heating, heat of reaction, specific heat, sensible heat, latent heat, waste heat, body heat, are not consistent with the strict thermodynamic meaning of th ...
... as: heat flow, heat addition, heat rejection, heat removal , heat gain, heat loss, heat storage, heat generation, electrical heating, resistance heating, heat of reaction, specific heat, sensible heat, latent heat, waste heat, body heat, are not consistent with the strict thermodynamic meaning of th ...
H 2 (g)
... m = mass of substance T = change in temperature (Tf – Ti) Cp = specific heat of substance ...
... m = mass of substance T = change in temperature (Tf – Ti) Cp = specific heat of substance ...
Tutorial Questions
... a pressure increase of 1 GPa. What will be its new temperature in relation to its initial temperature? You can make use of the fact that CP for Al is 24 Jmol-1K-1 (about 3R) and its volume expansivity () is 3 x 10-6 K-1. The density of Al is 2.7 x 103 kgm-3, and its atomic weight is 27 gmol-1. (4) ...
... a pressure increase of 1 GPa. What will be its new temperature in relation to its initial temperature? You can make use of the fact that CP for Al is 24 Jmol-1K-1 (about 3R) and its volume expansivity () is 3 x 10-6 K-1. The density of Al is 2.7 x 103 kgm-3, and its atomic weight is 27 gmol-1. (4) ...
File
... • is the enthalpy change for the formation of one mole of the substance from its elements, at standard pressure (1 atm) and a specified temperature (25°C) H2(g) + ½ O2(g) → H2O(l) C (graphite) + 2H2(g) → CH4(g) ...
... • is the enthalpy change for the formation of one mole of the substance from its elements, at standard pressure (1 atm) and a specified temperature (25°C) H2(g) + ½ O2(g) → H2O(l) C (graphite) + 2H2(g) → CH4(g) ...
Lecture25-12
... When 1 g of water boils at 100o C under 1 Atm. The volume of the steam at 100o C is 1671 cm3. Find the work done in the expansion and calculate the change in internal energy of the system ...
... When 1 g of water boils at 100o C under 1 Atm. The volume of the steam at 100o C is 1671 cm3. Find the work done in the expansion and calculate the change in internal energy of the system ...
Chemical Thermodynamics John Murrell Introduction
... is through thermodynamics that we define commonly used terms like heat, work, energy, and temperature. We also define some less commonly used words like entropy, heat capacity, and free energy. In fact, the whole concept of energy, and the relationship between one form of energy and another, was not ...
... is through thermodynamics that we define commonly used terms like heat, work, energy, and temperature. We also define some less commonly used words like entropy, heat capacity, and free energy. In fact, the whole concept of energy, and the relationship between one form of energy and another, was not ...
The Mayer-Joule Principle: The Foundation of
... As early as the 1620s, Bacon and Galileo (separately) hypothesized that heat was a consequence of the microscopic motion of the invisible particles that made up the hot body.9 However it was impossible to describe and relate this motion to any Newtonian dynamic quantity. In the mid-18th century a se ...
... As early as the 1620s, Bacon and Galileo (separately) hypothesized that heat was a consequence of the microscopic motion of the invisible particles that made up the hot body.9 However it was impossible to describe and relate this motion to any Newtonian dynamic quantity. In the mid-18th century a se ...