Mechanical Engineering (Electrical Branch)
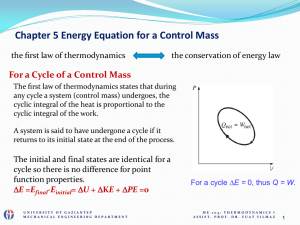
... All spontaneous process are irreversible process. Irreversible process cannot be shown on diagrams. They are shown as dotted lines. For example; heat transfer through finite temperature difference, free expansion. A system will be in a state of thermodynamic equilibrium if the conditions for the f ...
... All spontaneous process are irreversible process. Irreversible process cannot be shown on diagrams. They are shown as dotted lines. For example; heat transfer through finite temperature difference, free expansion. A system will be in a state of thermodynamic equilibrium if the conditions for the f ...
q 2 - q 1
... spontaneous change decreases with the occurrence of the natural process ( a change from non equilibrium state to equilibrium state ) until equilibrium is attained at which the capacity of the system for spontaneous change will be exhausted ; thus, if the process proceeds under infinitesimally small ...
... spontaneous change decreases with the occurrence of the natural process ( a change from non equilibrium state to equilibrium state ) until equilibrium is attained at which the capacity of the system for spontaneous change will be exhausted ; thus, if the process proceeds under infinitesimally small ...
Second Law of Thermodynamics
... 2) Clausius’s postulate: A transformation that permits heat transfer from a cold body to a hot body is impossible (p. 53, Tsonis). Recall that the First Law does not address the possibility of transformations; it only quantifies them, even if they are impossible. (Think of the First Law as the smart ...
... 2) Clausius’s postulate: A transformation that permits heat transfer from a cold body to a hot body is impossible (p. 53, Tsonis). Recall that the First Law does not address the possibility of transformations; it only quantifies them, even if they are impossible. (Think of the First Law as the smart ...
Unit II - Chemical Thermodynamics
... Enthalpy (H) is related with internal energy (E) as H = E + PV -------------------------(2) ...
... Enthalpy (H) is related with internal energy (E) as H = E + PV -------------------------(2) ...
Session 15 Thermodynamics
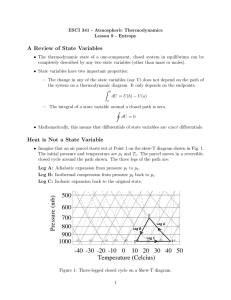
... We can change the state of the gas in various ways: by some combination of heating it or doing work on it. The top diagram in this section illustrates how we might change the temperature and pressure to those we are aiming for on the right, by applying heat in a fixed volume. In this case we supply ...
... We can change the state of the gas in various ways: by some combination of heating it or doing work on it. The top diagram in this section illustrates how we might change the temperature and pressure to those we are aiming for on the right, by applying heat in a fixed volume. In this case we supply ...
3.1 Thermal concepts (PPT)
... with the kinetic and potential energies of the molecules, i.e. how fast the molecules are vibrating and their chemical bonds. Heat goes from objects with high temperature to low temperature, not high thermal energy to low thermal energy. For example, a massive glacier will have more total thermal en ...
... with the kinetic and potential energies of the molecules, i.e. how fast the molecules are vibrating and their chemical bonds. Heat goes from objects with high temperature to low temperature, not high thermal energy to low thermal energy. For example, a massive glacier will have more total thermal en ...
Section 3 Entropy and Classical Thermodynamics
... 3.1.1 The Second Law of Thermodynamics There are various statements of the second law of thermodynamics. These must obviously be logically equivalent. In the spirit of our approach we shall adopt the following statement: • There exists an extensive function of state called entropy, such that in any ...
... 3.1.1 The Second Law of Thermodynamics There are various statements of the second law of thermodynamics. These must obviously be logically equivalent. In the spirit of our approach we shall adopt the following statement: • There exists an extensive function of state called entropy, such that in any ...
Calorimetry

Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transfer associated with changes of its state due for example to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat and the Greek word μέτρον (metron), meaning measure. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry.Indirect Calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The Dynamic Energy Budget theory explains why this procedure is correct. Heat generated by living organisms may also be measured by direct calorimetry, in which the entire organism is placed inside the calorimeter for the measurement.A widely used modern instrument is the differential scanning calorimeter, a device which allows thermal data to be obtained on small amounts of material. It involves heating the sample at a controlled rate and recording the heat flow either into or from the specimen.












![BTD QUESTION BANK[1].](http://s1.studyres.com/store/data/009330461_1-f5de3108f7a7a17ebe3a8cbd391865db-300x300.png)