The Laws of Thermodinamics
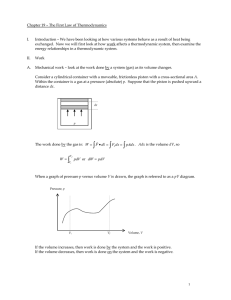
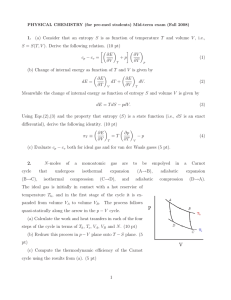
... W= -P ΔV –can be used to calculate the work done on the system only when the pressure of the gas remain ct. during the expansion or compression ISOBARIC PROCESS- a process in which the pressure remain constant • The area under the graph in a PV diagram is equal in magnitude to do work done on a gas ...
... W= -P ΔV –can be used to calculate the work done on the system only when the pressure of the gas remain ct. during the expansion or compression ISOBARIC PROCESS- a process in which the pressure remain constant • The area under the graph in a PV diagram is equal in magnitude to do work done on a gas ...
GasLawTheory
... GAS PRESSURE: The derivation of P = ghd Barometer measures atmospheric pressure – why mercury not water? (0.760 m) (13591.5 kg/m3) (9.80665m/s2) = 101325 N/m2 = 101.325 kPa ...
... GAS PRESSURE: The derivation of P = ghd Barometer measures atmospheric pressure – why mercury not water? (0.760 m) (13591.5 kg/m3) (9.80665m/s2) = 101325 N/m2 = 101.325 kPa ...
PHYS 1220, Engineering Physics, Chapter 19 – The First Law of
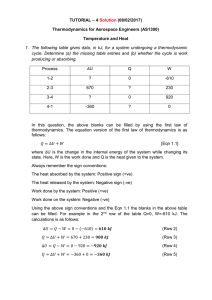
... - What is a “status” of a system? • A status is to describe the states of a system. It does not depend on the history of the system, rather it only depends on its thermodynamic state (e.g. temperature, volume, pressure, number of molecules etc.) - What is a “process”? • A process is to describe how ...
... - What is a “status” of a system? • A status is to describe the states of a system. It does not depend on the history of the system, rather it only depends on its thermodynamic state (e.g. temperature, volume, pressure, number of molecules etc.) - What is a “process”? • A process is to describe how ...
Themodynamic notes section 6.1
... • The entropy of a system not in thermal equilibrium will increase. • The entropy of a system approaches a constant value as the system approaches absolute zero. ...
... • The entropy of a system not in thermal equilibrium will increase. • The entropy of a system approaches a constant value as the system approaches absolute zero. ...
Test Thermodynamics Solutions
... property of all thermodynamics systems. When 2 bodies (A and B) are in thermal equilibrium (same temperature) with a third body (C), then all bodies (AC, BC, AB) are in equilibrium with ...
... property of all thermodynamics systems. When 2 bodies (A and B) are in thermal equilibrium (same temperature) with a third body (C), then all bodies (AC, BC, AB) are in equilibrium with ...
Relationships Between Heat and Work
... • Internal energy is constant in a constanttemperature process • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The ballo ...
... • Internal energy is constant in a constanttemperature process • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The ballo ...
$doc.title
... Thus, internal energy is introduced by the first law, and is related to the concepts of heat and work. The first law is just another way of stating the law of conservation of energy and reflects the results of many experiments on the work performed by or on a system, the heat added or subtracted, an ...
... Thus, internal energy is introduced by the first law, and is related to the concepts of heat and work. The first law is just another way of stating the law of conservation of energy and reflects the results of many experiments on the work performed by or on a system, the heat added or subtracted, an ...
Heat Chapter 12: Thermodynamics
... process can naturally or spontaneously take place. • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Ther ...
... process can naturally or spontaneously take place. • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Ther ...
Verdana 30 pt
... volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
Section 11
... can be done by a gas in the cylinder if the gas exerts a constant pressure of 7.5 x 105 Pa on the piston and moves the piston a distance of 0.040 m? ...
... can be done by a gas in the cylinder if the gas exerts a constant pressure of 7.5 x 105 Pa on the piston and moves the piston a distance of 0.040 m? ...
1. (a) Consider that an entropy S is as function of temperature T and
... (c) Evaluate cp − cv both for ideal gas and for van der Waals gases (5 pt). ...
... (c) Evaluate cp − cv both for ideal gas and for van der Waals gases (5 pt). ...
Problem set #2: 5
... 5.20 One mole of CO is initially contained on one-half of a well-insulated, rigid tank. Its temperature is 500oK. The other half of the tank is initially at vacuum. A diaphragm separates the two compartments. Each compartment has a volume of 1 L. Suddenly, the diaphragm ruptures. Use the van der Waa ...
... 5.20 One mole of CO is initially contained on one-half of a well-insulated, rigid tank. Its temperature is 500oK. The other half of the tank is initially at vacuum. A diaphragm separates the two compartments. Each compartment has a volume of 1 L. Suddenly, the diaphragm ruptures. Use the van der Waa ...
ME 433 Combustion Engine Systems
... 1. With what name/nickname do you prefer to be addressed? 2. What is your hometown/country? 3. What degree are you pursuing and when do you plan to graduate? 4. What previous experience do you have with combustion engines? 5. What grades did you receive in the following engineering science courses: ...
... 1. With what name/nickname do you prefer to be addressed? 2. What is your hometown/country? 3. What degree are you pursuing and when do you plan to graduate? 4. What previous experience do you have with combustion engines? 5. What grades did you receive in the following engineering science courses: ...
Atomic Structure
... 1. Consider the human body as a system and apply the first law of thermodynamics to it. We know that over any given period of sufficient length (say one day), there will be a net heat flow from the body (i.e. Q is negative) and the body will do some external work on its surroundings (i.e. W is posit ...
... 1. Consider the human body as a system and apply the first law of thermodynamics to it. We know that over any given period of sufficient length (say one day), there will be a net heat flow from the body (i.e. Q is negative) and the body will do some external work on its surroundings (i.e. W is posit ...