![Physics 322 Final Exam Study Guide (2015) [Pages 4 Only]](http://s1.studyres.com/store/data/007969504_1-e89a1630d6e27466a3e33b80f7e23b58-300x300.png)
Physics 322 Final Exam Study Guide (2015) [Pages 4 Only]
... 3. Energy Quantization and Spectral Lines in Hydrogen a. Know the basic characteristics of the hydrogen spectra and why it strongly suggests that the allowed energy levels of the electron in the hydrogen atom are quantized. b. Given the expression for the energy levels of the hydrogen atom, be able ...
... 3. Energy Quantization and Spectral Lines in Hydrogen a. Know the basic characteristics of the hydrogen spectra and why it strongly suggests that the allowed energy levels of the electron in the hydrogen atom are quantized. b. Given the expression for the energy levels of the hydrogen atom, be able ...
Download PDF
... In Eq. (2), I 0 ¼ jU 0 j2 and I 0 0 ¼ jU 0 0 j2 , where U 0 and U 0 0 represent, respectively, the incident plane wave and the unscattered field that passed through the slice, as illustrated in Fig. 1. Throughout the derivations, we ignore the effects of absorption. The field after the tissue slice, ...
... In Eq. (2), I 0 ¼ jU 0 j2 and I 0 0 ¼ jU 0 0 j2 , where U 0 and U 0 0 represent, respectively, the incident plane wave and the unscattered field that passed through the slice, as illustrated in Fig. 1. Throughout the derivations, we ignore the effects of absorption. The field after the tissue slice, ...
Isotope Shift of Hydrogen and Deuterium
... 4. Small corrections originating from quantum electrodynamics due to vacuum polarisation and zero point fluctuations of the radiation. These effects cause the "Lamb-shift". For further study of the effects mentioned in points 3 and 4, one is referred to [2]. Concerning the fine structure it can be s ...
... 4. Small corrections originating from quantum electrodynamics due to vacuum polarisation and zero point fluctuations of the radiation. These effects cause the "Lamb-shift". For further study of the effects mentioned in points 3 and 4, one is referred to [2]. Concerning the fine structure it can be s ...
THERMOCHEMISTRY ENERGETICS/ENTHALPY
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
Further Quantum Mechanics: Problem Set 2. Trinity term weeks 1 – 2
... now have of time-dependent problems in quantum mechanics. If you have not attempted it previously, you should do so. In the β decay H3 (1 proton + 2 neutrons in the nucleus) → (He3 )+ (2 protons + 1 neutron in the nucleus), the emitted electron has a kinetic energy of 16 keV. We will consider the ef ...
... now have of time-dependent problems in quantum mechanics. If you have not attempted it previously, you should do so. In the β decay H3 (1 proton + 2 neutrons in the nucleus) → (He3 )+ (2 protons + 1 neutron in the nucleus), the emitted electron has a kinetic energy of 16 keV. We will consider the ef ...
Chapter 7:
... •fibers usually constructed with variable refractive index and light is sent down the central core, which is surrounded by a material with a lower refractive index. •Light deviating from a straight path is totally internally reflected and hence remains in the core. ...
... •fibers usually constructed with variable refractive index and light is sent down the central core, which is surrounded by a material with a lower refractive index. •Light deviating from a straight path is totally internally reflected and hence remains in the core. ...
Modern physics
... wavelength λ=5.53·10-2 nm is scattered and detected at an angle of 35o. Find the fractional shift in the wavelength of the scattered X-ray. Solution: If λ is the incoming wavelength and λ’ is the wavelength of the scattered X ray, then the fractional change in wavelength is given by: ...
... wavelength λ=5.53·10-2 nm is scattered and detected at an angle of 35o. Find the fractional shift in the wavelength of the scattered X-ray. Solution: If λ is the incoming wavelength and λ’ is the wavelength of the scattered X ray, then the fractional change in wavelength is given by: ...
Key concepts of chemistry from high school chemistry
... An ion is an atom bearing a charge due to an imbalance in its number of protons and electrons. Since the very light electrons spin about the nucleus, electrons can be gained or lost by ato ...
... An ion is an atom bearing a charge due to an imbalance in its number of protons and electrons. Since the very light electrons spin about the nucleus, electrons can be gained or lost by ato ...
Are diglycolamide ligands hard or soft Lewis bases?
... The conclusion on the greater covalency of M–Oamid than M–Oether bonds results as well from quantum-mechanical bond analysis in the [M(TEDGA)3]3+ complexes: e.g. from Wiberg bond indices; from QTAIM parameters of the M–O bonds (e.g. electron densities, ρb, in the Bond Central Point) etc.; cf. the o ...
... The conclusion on the greater covalency of M–Oamid than M–Oether bonds results as well from quantum-mechanical bond analysis in the [M(TEDGA)3]3+ complexes: e.g. from Wiberg bond indices; from QTAIM parameters of the M–O bonds (e.g. electron densities, ρb, in the Bond Central Point) etc.; cf. the o ...
Reaction of potassium atoms with oriented bromotrifluoromethane
... adiabatic transitions into this field where they are now oriented with respect to the relative velocity for K CF3Br collisions. (CF31was studied as a comparison and the field plates were not realigned to account for the different CF31 speed, a correction of about 2 O . ) The direction of the molecul ...
... adiabatic transitions into this field where they are now oriented with respect to the relative velocity for K CF3Br collisions. (CF31was studied as a comparison and the field plates were not realigned to account for the different CF31 speed, a correction of about 2 O . ) The direction of the molecul ...
electron
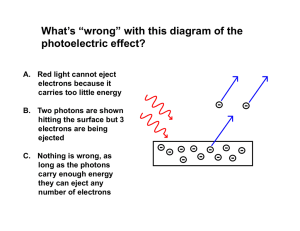
... What’s “wrong” with this diagram of the photoelectric effect? A. Red light cannot eject electrons because it carries too little energy B. Two photons are shown hitting the surface but 3 electrons are being ejected C. Nothing is wrong, as long as the photons carry enough energy they can eject any ...
... What’s “wrong” with this diagram of the photoelectric effect? A. Red light cannot eject electrons because it carries too little energy B. Two photons are shown hitting the surface but 3 electrons are being ejected C. Nothing is wrong, as long as the photons carry enough energy they can eject any ...
Paper
... The Letter by Deng et al. [1] presents an analytic theoretical description of matter-wave superradiance [2] which claims to go beyond previous theoretical frameworks. I show here that the theory presented in this Letter is not a description of superradiance per se, but rather an elegant perturbative ...
... The Letter by Deng et al. [1] presents an analytic theoretical description of matter-wave superradiance [2] which claims to go beyond previous theoretical frameworks. I show here that the theory presented in this Letter is not a description of superradiance per se, but rather an elegant perturbative ...
honors chem 6 day review packet
... Potassium Sulfur Cobalt P−3 Mg+2 Valence electrons are electrons in the outer shell. Draw the dot diagrams for the following: Potassium ...
... Potassium Sulfur Cobalt P−3 Mg+2 Valence electrons are electrons in the outer shell. Draw the dot diagrams for the following: Potassium ...
4.1Atoms and Isotopes
... An atom is composed of a central nucleus which consists of protons and neutrons, along with orbiting electrons that exist within ‘clouds’ or orbitals. These protons, neutrons, and electrons are commonly known as SUB-ATOMIC PARTICLES. ...
... An atom is composed of a central nucleus which consists of protons and neutrons, along with orbiting electrons that exist within ‘clouds’ or orbitals. These protons, neutrons, and electrons are commonly known as SUB-ATOMIC PARTICLES. ...
Slide 1
... 1. All matter is made of indivisible and indestructible atoms. 2. All atoms of a given element are identical in their physical and chemical properties. 3. Atoms of different elements differ in their physical and chemical properties. 4. Atoms of different elements combine in simple whole-number ratio ...
... 1. All matter is made of indivisible and indestructible atoms. 2. All atoms of a given element are identical in their physical and chemical properties. 3. Atoms of different elements differ in their physical and chemical properties. 4. Atoms of different elements combine in simple whole-number ratio ...
CHEM-UA 127: Advanced General Chemistry I
... as spring-like or Hooke’s law type forces. This is true provided the energy is not too high. Of course, at very high energy, the bond reaches its dissociation limit, and the forces deviate considerably from Hooke’s law. It is for this reason that it is useful to consider the quantum mechanics of a h ...
... as spring-like or Hooke’s law type forces. This is true provided the energy is not too high. Of course, at very high energy, the bond reaches its dissociation limit, and the forces deviate considerably from Hooke’s law. It is for this reason that it is useful to consider the quantum mechanics of a h ...
Einstein`s paper is a “bold, not to say reckless, hypothesis…which
... How do we know the atomic scale structure of matter around us? A crystal is a very large number of atoms or molecules arranged in a periodic fashion. It acts like a grating with an extremely large number (~Avagadro’s number) of units that diffract waves coherently. Every crystal has its own “signatu ...
... How do we know the atomic scale structure of matter around us? A crystal is a very large number of atoms or molecules arranged in a periodic fashion. It acts like a grating with an extremely large number (~Avagadro’s number) of units that diffract waves coherently. Every crystal has its own “signatu ...
Rayleigh scattering by gas molecules: why is the sky blue?
... In order to compare our measurements of scattered light power at different angles with theory, we need to use the concept of solid angle to quantify how much light our detector will detect given its distance from the source and its area. You will probably have come across solid angles in other conte ...
... In order to compare our measurements of scattered light power at different angles with theory, we need to use the concept of solid angle to quantify how much light our detector will detect given its distance from the source and its area. You will probably have come across solid angles in other conte ...
SAMPLE PAPER – III
... Downloaded from www.studiestoday.com expression for the angle of deviation relating i, e and A (angle of the prism). If the ray undergoes a minimum deviation of 30° then what is the refractive index of the material of the prism? ...
... Downloaded from www.studiestoday.com expression for the angle of deviation relating i, e and A (angle of the prism). If the ray undergoes a minimum deviation of 30° then what is the refractive index of the material of the prism? ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.