Chapter 5 The Wavelike - UCF College of Sciences
... If we know the momentum p of the particle as function of x, we can calculate the expectation value ‹p›. However, it is impossible in principle to find p as function of x since, according to uncertainty principle, both p and x can not be determined at the same time. To find ‹p› we need to know the di ...
... If we know the momentum p of the particle as function of x, we can calculate the expectation value ‹p›. However, it is impossible in principle to find p as function of x since, according to uncertainty principle, both p and x can not be determined at the same time. To find ‹p› we need to know the di ...
Answers
... ____ a) only the most massive reactant will be used up ____ b) neither of the reactants will be used up ____ c) both of the reactants will be used up ____ d) only the least massive reactant will be used up 35) The limiting reactant in a completed chemical reaction will be the substance ____ a) used ...
... ____ a) only the most massive reactant will be used up ____ b) neither of the reactants will be used up ____ c) both of the reactants will be used up ____ d) only the least massive reactant will be used up 35) The limiting reactant in a completed chemical reaction will be the substance ____ a) used ...
CST Review Part 2
... b. Students know the quantity on mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. c. Students know one mole equals 6.02 x 1023 particles (atoms or molecules). d. Students know how to determine the molar mass of a molecule from its chemical formula and a table o ...
... b. Students know the quantity on mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. c. Students know one mole equals 6.02 x 1023 particles (atoms or molecules). d. Students know how to determine the molar mass of a molecule from its chemical formula and a table o ...
Bal Equations notes.cwk (WP)
... You may start with any element or complex ion you like, however it may be easier to start with the greatest number. In this example we can start with calcium or sodium as both have 3 on one side of the equation. Lets start with sodium since it is first. Na3As (aq) has 3 Na. We must therefore have 3 ...
... You may start with any element or complex ion you like, however it may be easier to start with the greatest number. In this example we can start with calcium or sodium as both have 3 on one side of the equation. Lets start with sodium since it is first. Na3As (aq) has 3 Na. We must therefore have 3 ...
Word - chemmybear.com
... Example: 2Fe°(s) + 3Cl2(g) + heat 2FeCl3(s) 2. When you identify an oxidation product, make certain you also have a reduction product. Ex: “Free halogens + dilute OH- hypohalite ions," the halide ions (such as Cl-) as a product are required for full credit. [industrial process: making bleach] Cl2(g) ...
... Example: 2Fe°(s) + 3Cl2(g) + heat 2FeCl3(s) 2. When you identify an oxidation product, make certain you also have a reduction product. Ex: “Free halogens + dilute OH- hypohalite ions," the halide ions (such as Cl-) as a product are required for full credit. [industrial process: making bleach] Cl2(g) ...
IB Chemistry Online EQ_Ans
... There is a decrease in ionic radii from P3−, S2− to Cl−: all the ions have the electron arrangement of 2, 8, 8 (i.e. they are isoelectronic); however, there is a progressive increase in the nuclear charge due to the additional protons: the phosphide ion has 15 protons, the sulfide ion has 16 protons ...
... There is a decrease in ionic radii from P3−, S2− to Cl−: all the ions have the electron arrangement of 2, 8, 8 (i.e. they are isoelectronic); however, there is a progressive increase in the nuclear charge due to the additional protons: the phosphide ion has 15 protons, the sulfide ion has 16 protons ...
Limiting Reactant WS with Answers
... 11) 1.000 g of vanadium (V) is mixed with 8.000 g of bromine (Br2). After the elements react, some bromine is left over, along with a single compound that contains the two elements. The excess bromine is removed and allowed to react with excess sodium sulfite and excess sodium hydroxide, producing a ...
... 11) 1.000 g of vanadium (V) is mixed with 8.000 g of bromine (Br2). After the elements react, some bromine is left over, along with a single compound that contains the two elements. The excess bromine is removed and allowed to react with excess sodium sulfite and excess sodium hydroxide, producing a ...
Polarization of light II
... beam will contain the s component only. In this experiment , you will study the variation of intensity as a function of angle of incidence for p as well s polarized light and will measure the Brewster angle for air and glass interface and hence refractive index for glass can be estimated from eq (5) ...
... beam will contain the s component only. In this experiment , you will study the variation of intensity as a function of angle of incidence for p as well s polarized light and will measure the Brewster angle for air and glass interface and hence refractive index for glass can be estimated from eq (5) ...
Phase-separation in ion-containing mixtures in electric fields
... separates into its components when put under the In the absence of field (mixed state) and under coninfluence of an electric field in some reasonable condi- stant applied external stress, the mixture will have tions. The dissociated ions in the solution are impor- the homogeneous viscosity ηm , and ...
... separates into its components when put under the In the absence of field (mixed state) and under coninfluence of an electric field in some reasonable condi- stant applied external stress, the mixture will have tions. The dissociated ions in the solution are impor- the homogeneous viscosity ηm , and ...
Honors Directed Study Abstract - PS 303
... the PSI High Intensity Proton Accelerator, in operation from 1974 to the present and using a proton beam and graphite target that releases neutrons and used to produce mesons and neutrons [5]; the TRIUMF Cyclotron, also in operation from 1974 to the present and using proton beams; the Mainz Microtro ...
... the PSI High Intensity Proton Accelerator, in operation from 1974 to the present and using a proton beam and graphite target that releases neutrons and used to produce mesons and neutrons [5]; the TRIUMF Cyclotron, also in operation from 1974 to the present and using proton beams; the Mainz Microtro ...
A high resolution ion microscope for cold atoms
... Rydberg atoms (atoms in states of high principal quantum number [16]) shall be imaged, the imaging techniques cannot always be applied directly. In case of earth alkali Rydberg atoms, the second valence electron can be used for such a closed cycle [17]. But with the more commonly used alkali Rydberg ...
... Rydberg atoms (atoms in states of high principal quantum number [16]) shall be imaged, the imaging techniques cannot always be applied directly. In case of earth alkali Rydberg atoms, the second valence electron can be used for such a closed cycle [17]. But with the more commonly used alkali Rydberg ...
Covalent bonding
... E.g., suppose that two charges 1+ and 1- are separated by a distance of 1.00 Å: calculate µ Note that dipole moments are generally reported in units of ...
... E.g., suppose that two charges 1+ and 1- are separated by a distance of 1.00 Å: calculate µ Note that dipole moments are generally reported in units of ...
chapter3 - AlvarezHChem
... a. sum all atoms of each type on a side, even if an element is in more than one substance b. work from left to right to stay organized c. if a polyatomic ion is present in the same form on both sides it can be counted as a unit rather than as individual elements d. look to balance H’s and O’s last i ...
... a. sum all atoms of each type on a side, even if an element is in more than one substance b. work from left to right to stay organized c. if a polyatomic ion is present in the same form on both sides it can be counted as a unit rather than as individual elements d. look to balance H’s and O’s last i ...
2.ATOMS, MOLECULES, AND IONS
... they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two elements with the correct ratio of atoms, 1:2; therefore, it must be CO2. ...
... they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two elements with the correct ratio of atoms, 1:2; therefore, it must be CO2. ...
Biol 1406 notes Ch 2 8thed
... o An electron cannot exist between these fixed locations. The different states of potential energy that the electrons of an atom can have are called electron shells. o The first shell, closest to the nucleus, has the lowest potential energy. o Electrons in outer shells have higher potential energy ...
... o An electron cannot exist between these fixed locations. The different states of potential energy that the electrons of an atom can have are called electron shells. o The first shell, closest to the nucleus, has the lowest potential energy. o Electrons in outer shells have higher potential energy ...
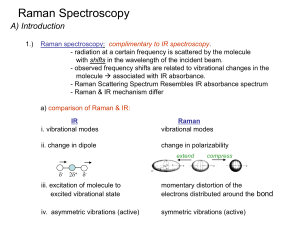
Raman spectroscopy
... - Raman does not “see” many common polar solvents can use with aqueous samples – advantage over IR ...
... - Raman does not “see” many common polar solvents can use with aqueous samples – advantage over IR ...
N - Unibas Chemie
... Nk, specified as {N0, N1, ...}, and which has E0, E1, ... energies. A system of molecules has a very large number of instantaneous configurations, which fluctuate with time due to the populations change: {N, 0, 0, 0, ...}, {N-1, 1, 0, 0, ...}, {N-1, 0, 1, 0, ...}, {N-2, 2, 0, 0, ...}, {N-2, 0, 2, 0, ...
... Nk, specified as {N0, N1, ...}, and which has E0, E1, ... energies. A system of molecules has a very large number of instantaneous configurations, which fluctuate with time due to the populations change: {N, 0, 0, 0, ...}, {N-1, 1, 0, 0, ...}, {N-1, 0, 1, 0, ...}, {N-2, 2, 0, 0, ...}, {N-2, 0, 2, 0, ...
A simple and effective approach to calculate the energy of complex
... is based on the Z −1 expansion due to Layzer [9] and originated in works of Hylleraas on the ground state of He. On one hand, this is an interesting exercise for nongraduate students. We show as to attain data even for complex atoms that are well compared with the experiment in diverse cases: bindin ...
... is based on the Z −1 expansion due to Layzer [9] and originated in works of Hylleraas on the ground state of He. On one hand, this is an interesting exercise for nongraduate students. We show as to attain data even for complex atoms that are well compared with the experiment in diverse cases: bindin ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.