Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _____. A) protons B) electrons when neutral ...
... B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _____. A) protons B) electrons when neutral ...
Chemical Bonding and Molecular Structure Bonding: Ionic vs
... • IMPORTANT! The sum of all the FC for a species or ion MUST equal the net charge on the species! Example: Calculate the FC on each atom in CN-: FC can help when drawing Lewis Structures 1. FC on each atom should be as small as possible 2. (-) FC should appear on the most electronegative atoms, (+) ...
... • IMPORTANT! The sum of all the FC for a species or ion MUST equal the net charge on the species! Example: Calculate the FC on each atom in CN-: FC can help when drawing Lewis Structures 1. FC on each atom should be as small as possible 2. (-) FC should appear on the most electronegative atoms, (+) ...
Atomic Weights Average Atomic Masses
... Atomic Weights Average Atomic Masses • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Ato ...
... Atomic Weights Average Atomic Masses • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Ato ...
Qualitative Solutions of the TISE
... have the longest (average) wavelength and therefore the lowest energy; the twobump (one-node) wavefunction will have the second-longest (average) wavelength and therefore the second-lowest energy; and so on. To determine the numerical values of these energies, of course, requires doing some actual c ...
... have the longest (average) wavelength and therefore the lowest energy; the twobump (one-node) wavefunction will have the second-longest (average) wavelength and therefore the second-lowest energy; and so on. To determine the numerical values of these energies, of course, requires doing some actual c ...
Name: (1 of 2) Math Set # 13 Protons,
... The number of protons is ALWAYS the same for an atom of a specific element. Germanium ALWAYS has 32 protons. If you add a proton it is no longer Germanium but becomes Arsenic. ...
... The number of protons is ALWAYS the same for an atom of a specific element. Germanium ALWAYS has 32 protons. If you add a proton it is no longer Germanium but becomes Arsenic. ...
Chem Review
... 8. Which of the following is not true about electrons? a. They take part in bonding b. They like to pair up c. They have equal attraction to all elements d. They fill the orbitals in a specific order e. They can be transferred or shared 9. The order electrons fill orbitals is: a. 1s 2s 3s 3g 4s 5f 6 ...
... 8. Which of the following is not true about electrons? a. They take part in bonding b. They like to pair up c. They have equal attraction to all elements d. They fill the orbitals in a specific order e. They can be transferred or shared 9. The order electrons fill orbitals is: a. 1s 2s 3s 3g 4s 5f 6 ...
Franck-Hertz Effect in Mercury
... (Unbound states can have any energy.) The Hg atom normally will be in the lowest or ground state, with a valence electron occupancy designated by (6s)2 (two electrons in n=6, l = 0 single particle states). This has a spectroscopic designation of lS0, or (2S+1LJ), where S, L and J are system orbital, ...
... (Unbound states can have any energy.) The Hg atom normally will be in the lowest or ground state, with a valence electron occupancy designated by (6s)2 (two electrons in n=6, l = 0 single particle states). This has a spectroscopic designation of lS0, or (2S+1LJ), where S, L and J are system orbital, ...
Formulae and equations
... Get the correct formula for each species. Include the state symbols if necessary. Once you have obtained the correct formula of a species you must not change it to help balance an equation. ...
... Get the correct formula for each species. Include the state symbols if necessary. Once you have obtained the correct formula of a species you must not change it to help balance an equation. ...
Crystal Field Theory, gemstones and color
... The first structural feature we will investigate is the presence of the cyclosilicate motif. First, lets create Si-O tetrahedra for easier viewing. Under the “edit” menu, select “bonding,” and press the “+” to add a new type of bond. Form bonds between Si and O by settin ...
... The first structural feature we will investigate is the presence of the cyclosilicate motif. First, lets create Si-O tetrahedra for easier viewing. Under the “edit” menu, select “bonding,” and press the “+” to add a new type of bond. Form bonds between Si and O by settin ...
Photoelectron spectroscopy of jet
... first maximum positions (see Fig. 4) show a strong increase with n; these values converge slowly to an electron binding energy of about 2.5 to 3.0 eV, still being far away from the bulk work function (0 = 4.28 eV [22]). Beside the monotonous trend some outstanding high (n=4, 6, 9, 19, 22) and low (n ...
... first maximum positions (see Fig. 4) show a strong increase with n; these values converge slowly to an electron binding energy of about 2.5 to 3.0 eV, still being far away from the bulk work function (0 = 4.28 eV [22]). Beside the monotonous trend some outstanding high (n=4, 6, 9, 19, 22) and low (n ...
Effects of antioxidants for the degradation of flame
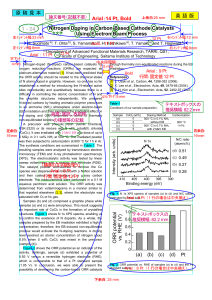
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
bond is
... • Any 2-atom molecule with a polar bond has a dipole moment. • The molecule will be polar ...
... • Any 2-atom molecule with a polar bond has a dipole moment. • The molecule will be polar ...
chemistry
... The possession or use of any communications device is strictly prohibited when taking this examination. If you have or use any communications device, no matter how briefly, your examination will be invalidated and no score will be calculated for you. This is a test of your knowledge of chemistry. Us ...
... The possession or use of any communications device is strictly prohibited when taking this examination. If you have or use any communications device, no matter how briefly, your examination will be invalidated and no score will be calculated for you. This is a test of your knowledge of chemistry. Us ...
"Reverse phase" PMMA-b-PS block copolymers as
... surface followed by processing shown in Figure 2 (right). (right) Other samples were: Si/Ta 5nm/Ru 50nm/CoPt 20nm/Ta 5nm (cap). Reactive ion etching of initial Si and Ta samples used only O2 while the magnetic samples employed a mixture of RIE with O2 and CF4 plus 5.5 min of ion beam etching. Struct ...
... surface followed by processing shown in Figure 2 (right). (right) Other samples were: Si/Ta 5nm/Ru 50nm/CoPt 20nm/Ta 5nm (cap). Reactive ion etching of initial Si and Ta samples used only O2 while the magnetic samples employed a mixture of RIE with O2 and CF4 plus 5.5 min of ion beam etching. Struct ...
2 day in-class guided inquiry exercise
... Let’s examine the structure of beryl in more detail. Open the CrystalMaker entitled “beryl.cmdf” on your laptop. You should see the unit cell, the axis system, and the unit cell contents. All of the atoms shown are positioned within the unit cell; verify that the stoichiometry of the mineral is corr ...
... Let’s examine the structure of beryl in more detail. Open the CrystalMaker entitled “beryl.cmdf” on your laptop. You should see the unit cell, the axis system, and the unit cell contents. All of the atoms shown are positioned within the unit cell; verify that the stoichiometry of the mineral is corr ...
Crystal Field Theory, gemstones and color
... Let’s examine the structure of beryl in more detail. Open the CrystalMaker entitled “beryl.cmdf” on your laptop. You should see the unit cell, the axis system, and the unit cell contents. All of the atoms shown are positioned within the unit cell; verify that the stoichiometry of the mineral is corr ...
... Let’s examine the structure of beryl in more detail. Open the CrystalMaker entitled “beryl.cmdf” on your laptop. You should see the unit cell, the axis system, and the unit cell contents. All of the atoms shown are positioned within the unit cell; verify that the stoichiometry of the mineral is corr ...
New high field magnet for neutron scattering at Hahn Meitner Institute
... return on the effort invested, as the relatively modest step from 11 T to 15 T did so convincingly in the past. The 15 T world record performance was achieved by the conventional design of placing two superconducting solenoids close to each other with a common axis. Between the solenoids there is a ...
... return on the effort invested, as the relatively modest step from 11 T to 15 T did so convincingly in the past. The 15 T world record performance was achieved by the conventional design of placing two superconducting solenoids close to each other with a common axis. Between the solenoids there is a ...
First Midterm Answer Key
... π-electrons are more polarizable than σ-electrons, even though N is less electronegative than O, the C-N triple bond has more π-electrons (2 π-bonds compared to 1), and thus the electrons are more polarized, the dipole moment is thus larger Question 7 (22 pts.) For the indicated localized molecular ...
... π-electrons are more polarizable than σ-electrons, even though N is less electronegative than O, the C-N triple bond has more π-electrons (2 π-bonds compared to 1), and thus the electrons are more polarized, the dipole moment is thus larger Question 7 (22 pts.) For the indicated localized molecular ...
2003
... macroscopic number of particles in the lowest quantum state. Condensate fragmentation, the macroscopic occupation of two or more quantum states, is usually prevented by interactions [16]. However, multiple condensates may exist in metastable situations. Let's assume that an equilibrium condensate ha ...
... macroscopic number of particles in the lowest quantum state. Condensate fragmentation, the macroscopic occupation of two or more quantum states, is usually prevented by interactions [16]. However, multiple condensates may exist in metastable situations. Let's assume that an equilibrium condensate ha ...
Directed Reading
... ______ 7. When iron reacts with oxygen to form rust, the reaction is an example of a a. physical property of oxygen. b. magnetic property of oxygen. c. chemical property of iron. d. physical property of iron. ______ 8. Which of the following is a chemical property of helium? a. Helium does not react ...
... ______ 7. When iron reacts with oxygen to form rust, the reaction is an example of a a. physical property of oxygen. b. magnetic property of oxygen. c. chemical property of iron. d. physical property of iron. ______ 8. Which of the following is a chemical property of helium? a. Helium does not react ...
Document
... benzene have six carbon atoms and six hydrogen atoms shaped in a ring. An atom can make one chemical bond for each valence electron. Bonds can also involve two or more valence electrons. ...
... benzene have six carbon atoms and six hydrogen atoms shaped in a ring. An atom can make one chemical bond for each valence electron. Bonds can also involve two or more valence electrons. ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.