The Chemical Composition of the Earth`s Original Atmosphere
... Composition given as volume percent for each gas excluding water. Water is given as percentage of total gases. ...
... Composition given as volume percent for each gas excluding water. Water is given as percentage of total gases. ...
Thermochemistry
... Energy put into a system may be converted to mechanical work. Work done on a system will increase its energy content. In the above example of the change of state of CO2, during the final step, (the expansion of the gas) work will be done by the gas on its surroundings. Suppose the “system” (gas in t ...
... Energy put into a system may be converted to mechanical work. Work done on a system will increase its energy content. In the above example of the change of state of CO2, during the final step, (the expansion of the gas) work will be done by the gas on its surroundings. Suppose the “system” (gas in t ...
Unit5C - OCCC.edu
... Example: Write the complete ionic and net ionic equations for the reaction. Which element is oxidized? What is the oxidizing ...
... Example: Write the complete ionic and net ionic equations for the reaction. Which element is oxidized? What is the oxidizing ...
Use the following answers for questions 10
... When the concentration of substance B in the reaction above is doubled, all other factors being held constant, it is found that the rate of the reaction remains unchanged. The most probable explanation for this observation is that (A) the order of the reaction with respect to substance B is 1 (B) s ...
... When the concentration of substance B in the reaction above is doubled, all other factors being held constant, it is found that the rate of the reaction remains unchanged. The most probable explanation for this observation is that (A) the order of the reaction with respect to substance B is 1 (B) s ...
CHEMISTRY SAMPLE PAPER - I
... (a) the reduction of a metal oxide is easier if the metal formed is in liquid state at the temperature of reducation. (b) the reduction of Cr2O3 with AI is thermodynamically feasible, yet it does not occur at room temperature. (c) pine oil is used in froth floatation method. 3 23. Explain the follow ...
... (a) the reduction of a metal oxide is easier if the metal formed is in liquid state at the temperature of reducation. (b) the reduction of Cr2O3 with AI is thermodynamically feasible, yet it does not occur at room temperature. (c) pine oil is used in froth floatation method. 3 23. Explain the follow ...
Final "I Can Statements" Answer Key
... _____10. Given a heating/cooling curve, I can determine which sections of the curve show changes in kinetic energy. On the graph, circle the sections that show a change in kinetic energy. _____11. I can state the temperature at which water freezes in oC and K. ...
... _____10. Given a heating/cooling curve, I can determine which sections of the curve show changes in kinetic energy. On the graph, circle the sections that show a change in kinetic energy. _____11. I can state the temperature at which water freezes in oC and K. ...
Document
... b) you need at least 0.250 moles of HNO3 to produce 0.500 moles of Cu(NO3)2. c) you need at least 2.00 moles of HNO3 to produce 0.500 moles of Cu(NO3)2. d) you need at least 2.00 moles of HNO3 to produce 1.00 moles of Cu(NO3)2. e) you need at least 2.00 moles of HNO3 to produce 2.00 moles of Cu(NO3) ...
... b) you need at least 0.250 moles of HNO3 to produce 0.500 moles of Cu(NO3)2. c) you need at least 2.00 moles of HNO3 to produce 0.500 moles of Cu(NO3)2. d) you need at least 2.00 moles of HNO3 to produce 1.00 moles of Cu(NO3)2. e) you need at least 2.00 moles of HNO3 to produce 2.00 moles of Cu(NO3) ...
Sample Final Questions Key/FS12
... The lines are created when the electrons make transitions from one level to another. Since Hydrogen has only one electron and since there are only so many allowable transitions, then the Hydrogen spectrum has the fewest lines. ...
... The lines are created when the electrons make transitions from one level to another. Since Hydrogen has only one electron and since there are only so many allowable transitions, then the Hydrogen spectrum has the fewest lines. ...
std 8 9 reviewanswers
... C A catalyst works by lowering the activation energy for the reaction 9. Chemical equilibrium is a dynamic process at the molecular level. As a basis for understanding this concept: a. Students know how to use Le Chatelier’s principle to predict the effect of changes in concentration, temperature, a ...
... C A catalyst works by lowering the activation energy for the reaction 9. Chemical equilibrium is a dynamic process at the molecular level. As a basis for understanding this concept: a. Students know how to use Le Chatelier’s principle to predict the effect of changes in concentration, temperature, a ...
Chemical Monitoring and Management by Ahmad Shah Idil
... Pressure: Increased pressure will produce more ammonia, also faster, but it will be expensive to build and maintain high-pressure equipment. The benefits of high pressure outweigh the costs, and so a pressure of 35 MPa is used. ...
... Pressure: Increased pressure will produce more ammonia, also faster, but it will be expensive to build and maintain high-pressure equipment. The benefits of high pressure outweigh the costs, and so a pressure of 35 MPa is used. ...
aq - HCC Learning Web
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
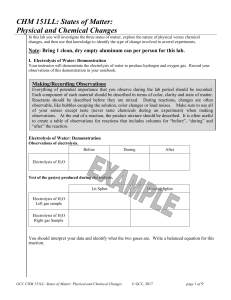
CHM 151LL: States of Matter: Physical and Chemical Changes
... Chemists in industry and research are interested in the changes substances undergo in order to provide better products to the marketplace and to discover new potentially useful substances. Chemical changes do change the composition of the atoms or molecules in the substance. They result in new subst ...
... Chemists in industry and research are interested in the changes substances undergo in order to provide better products to the marketplace and to discover new potentially useful substances. Chemical changes do change the composition of the atoms or molecules in the substance. They result in new subst ...
Production of Materials by Jason Yu #2
... Because very little ethylene is found in natural gas or crude oil, it must be produced from other hydrocarbons by a process known as ‘cracking’ Fractions used are higher molecular mass fractions for which there is less market demand. Products of cracking include short chain alkanes that can be used ...
... Because very little ethylene is found in natural gas or crude oil, it must be produced from other hydrocarbons by a process known as ‘cracking’ Fractions used are higher molecular mass fractions for which there is less market demand. Products of cracking include short chain alkanes that can be used ...
Example 1-2
... The solubility of a solute is the amount that can be dissolved in a given quantity of solvent at a given temperature. For example, the solubility of lead (II) nitrate is 56 g/100 mL at 20oC. The solubilities of ionic solids in water vary over a wide range of values. For convenience, we divide compou ...
... The solubility of a solute is the amount that can be dissolved in a given quantity of solvent at a given temperature. For example, the solubility of lead (II) nitrate is 56 g/100 mL at 20oC. The solubilities of ionic solids in water vary over a wide range of values. For convenience, we divide compou ...
Chemistry Entrance Material for Grade 11 to 12 Answer Key
... [-B-] The liquid continues to cool down. [-C-] The liquid may cool down initially, but then it will stay at the same temperature because the system has reached equilibrium. [-D-] The remaining liquid becomes hotter because evaporation needs heat. [-E-] None of the above Section №: 2 Solutions A solu ...
... [-B-] The liquid continues to cool down. [-C-] The liquid may cool down initially, but then it will stay at the same temperature because the system has reached equilibrium. [-D-] The remaining liquid becomes hotter because evaporation needs heat. [-E-] None of the above Section №: 2 Solutions A solu ...
by John Mu
... ones using special catalysts The catalyst used is called a zeolite If it is not decomposed entirely the Hydrocarbons are further decomposed by steam cracking − A mixture of alkanes with steam is passed through very hot metal tubes. ...
... ones using special catalysts The catalyst used is called a zeolite If it is not decomposed entirely the Hydrocarbons are further decomposed by steam cracking − A mixture of alkanes with steam is passed through very hot metal tubes. ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.