Topical KCSE Mock-Chemistry Answers(15 Schools)
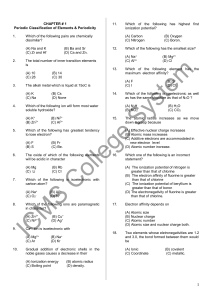
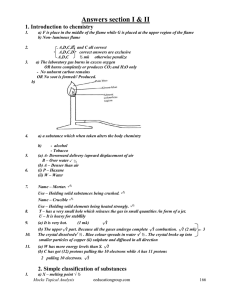
... (iii) Magnesium react with oxygen and nitrogen hence greater of fraction of air is used. (iv) (a) Blue litmus changed to red as remained red. The solution was acid due to phosphoric (b) Red litmus changed to blue as blue remained blue due to formation of basic magnesium hydroxide ammonia solution. ( ...
... (iii) Magnesium react with oxygen and nitrogen hence greater of fraction of air is used. (iv) (a) Blue litmus changed to red as remained red. The solution was acid due to phosphoric (b) Red litmus changed to blue as blue remained blue due to formation of basic magnesium hydroxide ammonia solution. ( ...
13- and 14-membered macrocyclic ligands containing
... same experimental conditions, and the corresponding values are also collected in Tables 1 and 2. The literature values for dota and dotp with the same metal ions are also listed for comparison reasons, however recommended values for these two ligands do not exist [36]. Indeed, the very high values o ...
... same experimental conditions, and the corresponding values are also collected in Tables 1 and 2. The literature values for dota and dotp with the same metal ions are also listed for comparison reasons, however recommended values for these two ligands do not exist [36]. Indeed, the very high values o ...
Modern inorganic chemistry
... corresponding to a particular energy state. The "orbit' model accurately interpreted the spectrum of hydrogen but was less successful for other elements. Hydrogen, the simplest atom, is made up of a proton (nucleus) and an electron. The electron normally exists in the lowest energy state £15 but may ...
... corresponding to a particular energy state. The "orbit' model accurately interpreted the spectrum of hydrogen but was less successful for other elements. Hydrogen, the simplest atom, is made up of a proton (nucleus) and an electron. The electron normally exists in the lowest energy state £15 but may ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... When making solution A, 25 cm3 of the NaOH solution reacts with 25 cm3 of the CH3COOH solution. This forms some ethanoate ions, CH3COO-(aq), and leaves some unreacted ethanoic acid molecules, CH3COOH. CH3COOH(aq) + OH−(aq) → CH3COO−(aq) + H2O(l) So, solution A contains a mixture of a weak acid, CH3C ...
... When making solution A, 25 cm3 of the NaOH solution reacts with 25 cm3 of the CH3COOH solution. This forms some ethanoate ions, CH3COO-(aq), and leaves some unreacted ethanoic acid molecules, CH3COOH. CH3COOH(aq) + OH−(aq) → CH3COO−(aq) + H2O(l) So, solution A contains a mixture of a weak acid, CH3C ...
advanced placement chemistry workbook and note set
... The name of a particular isotope is the element name plus its mass number. The name of the isotope on the far left in Figure 4 is carbon-12, the middle isotope is carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You c ...
... The name of a particular isotope is the element name plus its mass number. The name of the isotope on the far left in Figure 4 is carbon-12, the middle isotope is carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You c ...
CLUE - virtual laboratories
... combination that leads to the widespread public misunderstanding of chemical principles. How many times do we hear about “natural remedies, without drugs or chemicals,” despite the fact that everything is composed of chemicals and the most toxic chemicals known are natural products.2 A growing body ...
... combination that leads to the widespread public misunderstanding of chemical principles. How many times do we hear about “natural remedies, without drugs or chemicals,” despite the fact that everything is composed of chemicals and the most toxic chemicals known are natural products.2 A growing body ...
A Few Things You Might Want To Know
... Bond and molecule polarities are not discrete properties, i.e., they change gradually. The EN differences serve as rough guidelines only for the determination of bind polarity. ...
... Bond and molecule polarities are not discrete properties, i.e., they change gradually. The EN differences serve as rough guidelines only for the determination of bind polarity. ...
Chapter 8
... formed from reactant A, assuming it is the limiting reactant b) Find the number of moles of product that can be formed from reactant B, assuming it is the limiting reactant 3) Whichever number of moles of product is smallest corresponds to the limiting reactant. The other reactant is in excess. The ...
... formed from reactant A, assuming it is the limiting reactant b) Find the number of moles of product that can be formed from reactant B, assuming it is the limiting reactant 3) Whichever number of moles of product is smallest corresponds to the limiting reactant. The other reactant is in excess. The ...
Two-Electron Reduction of a Vanadium(V) Nitride by CO to Release
... bridging nitrido ligands have been shown to combine with CO while effecting 2e reduction of the ligated metal.12-16 Similarly, discrete metal complexes bearing oxo ligands have been shown to provide CO2 upon treatment with CO,17 and heterogeneous catalysis provides many examples of CO acting as a te ...
... bridging nitrido ligands have been shown to combine with CO while effecting 2e reduction of the ligated metal.12-16 Similarly, discrete metal complexes bearing oxo ligands have been shown to provide CO2 upon treatment with CO,17 and heterogeneous catalysis provides many examples of CO acting as a te ...
contact - DTU Kemi
... be added to the reaction mixture in the same reaction vessel from the outset. Neither catalyst will interfere with the job of the other, as the effect of the Brønsted acid catalysis will set in only after the ruthenium hydride catalytic process has created the relevant compound for the other catalys ...
... be added to the reaction mixture in the same reaction vessel from the outset. Neither catalyst will interfere with the job of the other, as the effect of the Brønsted acid catalysis will set in only after the ruthenium hydride catalytic process has created the relevant compound for the other catalys ...
Manual Physical Chemistry III
... molecules in their close vicinity only (zone of molecular interaction). In the bulk of the liquid, each molecule is attracted equally in all directions by the neighboring molecules; hence zero net force (Fig. 1). However, the molecules at the surface do not have other like molecules on all sides aro ...
... molecules in their close vicinity only (zone of molecular interaction). In the bulk of the liquid, each molecule is attracted equally in all directions by the neighboring molecules; hence zero net force (Fig. 1). However, the molecules at the surface do not have other like molecules on all sides aro ...
Chemistry Unit 1
... • explain the chemical properties of acidic oxides; • define basic oxides and give examples; • explain the chemical properties of basic oxides; • conduct experiments to distinguish acidic oxides from basic oxides; • compare and contrast acidic and basic oxides; • define amphoteric oxides and give ex ...
... • explain the chemical properties of acidic oxides; • define basic oxides and give examples; • explain the chemical properties of basic oxides; • conduct experiments to distinguish acidic oxides from basic oxides; • compare and contrast acidic and basic oxides; • define amphoteric oxides and give ex ...
chemical reactions and stoichiometry chemical reactions and
... Strategy: This problem looks complicated, so it is a good idea to apply the seven-step problem-solving method. 1. This is a stoichiometry problem (how much?), in which we are asked to find the mass of a reactant. 2. To visualize this kind of problem, we need a balanced chemical equation. Write the u ...
... Strategy: This problem looks complicated, so it is a good idea to apply the seven-step problem-solving method. 1. This is a stoichiometry problem (how much?), in which we are asked to find the mass of a reactant. 2. To visualize this kind of problem, we need a balanced chemical equation. Write the u ...
Here
... (d) van der Waals equation is a relation between the pressure, temperature and volume of a gas that accounts for the non‐zero size of the gas molecules and the attractive forces between them. (e) Gibbs free energy, G = H − TS, combines enthalpy and entropy to give a quantity which must decreas ...
... (d) van der Waals equation is a relation between the pressure, temperature and volume of a gas that accounts for the non‐zero size of the gas molecules and the attractive forces between them. (e) Gibbs free energy, G = H − TS, combines enthalpy and entropy to give a quantity which must decreas ...
class notes 4
... solid when the reactants switch partners. Must have a solid precipitate form or it won’t go. b. Acid-Base Reaction: An acid and a base are mixed and we get water and a salt, when the acid and base switch partners. Acid-base reactions always go. c. Gas-Evolution Reaction: Two aqueous solutions are mi ...
... solid when the reactants switch partners. Must have a solid precipitate form or it won’t go. b. Acid-Base Reaction: An acid and a base are mixed and we get water and a salt, when the acid and base switch partners. Acid-base reactions always go. c. Gas-Evolution Reaction: Two aqueous solutions are mi ...
CHEM 101 Fall 09 Final Exam (a)
... 12. What is the frequency (s-1) of a photon that has an energy of 4.38 × 10-18 J? a. 436 b. 6.61 × 1015 c. 1.45 × 10-16 d. 2.30 × 107 e. 1.31 × 10-9 13. Which answer shows all possible values of the second quantum number when n = 3? a. l = 0 b. l = 0, 1 c. l = 0, 1, 2 d. l = 0, 1, 2, 3 e. l = 0, 1, ...
... 12. What is the frequency (s-1) of a photon that has an energy of 4.38 × 10-18 J? a. 436 b. 6.61 × 1015 c. 1.45 × 10-16 d. 2.30 × 107 e. 1.31 × 10-9 13. Which answer shows all possible values of the second quantum number when n = 3? a. l = 0 b. l = 0, 1 c. l = 0, 1, 2 d. l = 0, 1, 2, 3 e. l = 0, 1, ...
Rhenium- and molybdenum-catalyzed dehydration reactions
... methyltrioxorhenium (CH3ReO3, MTO) to be an active catalyst in olefin metathesis,1 aldehyde olefination,2 and olefin epoxidation.3 In the 20 years after, the class of organorhenium oxides has found application in a very broad scope of catalytic reactions. One of the fields in which these organorheni ...
... methyltrioxorhenium (CH3ReO3, MTO) to be an active catalyst in olefin metathesis,1 aldehyde olefination,2 and olefin epoxidation.3 In the 20 years after, the class of organorhenium oxides has found application in a very broad scope of catalytic reactions. One of the fields in which these organorheni ...
Thermal Decomposition of the Non-Interstitial Hydrides for the
... the reservoir/storage mass), be inexpensive, have rapid kinetics for absorbing and desorbing H2 in the 25-120 °C temperature range, and store large quantities of hydrogen reversibly (a much more detailed “wish-list” is expanded-upon shortly). It is important to stress at the outset that, at present, ...
... the reservoir/storage mass), be inexpensive, have rapid kinetics for absorbing and desorbing H2 in the 25-120 °C temperature range, and store large quantities of hydrogen reversibly (a much more detailed “wish-list” is expanded-upon shortly). It is important to stress at the outset that, at present, ...
CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the 'kinetic model' of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the 'kinetic model' of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
PDF - mockies – Mockiesgateacademy
... Alchemy was a mixture of scientific investigation and mystical quest, with strands of philosophy from Greece, China, Egypt and Arabia mixed in. The main aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), an ...
... Alchemy was a mixture of scientific investigation and mystical quest, with strands of philosophy from Greece, China, Egypt and Arabia mixed in. The main aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), an ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... The synthesis of low molecular weight nickel (II) complexes mimicking superoxide dismutase (SOD) activity has been challenging for bioinorganic chemists and recently some complexes with high catalytic activity have been reported [1-3]. Nikel-contaning superoxide dismutase (NiSOD) has been isolated f ...
... The synthesis of low molecular weight nickel (II) complexes mimicking superoxide dismutase (SOD) activity has been challenging for bioinorganic chemists and recently some complexes with high catalytic activity have been reported [1-3]. Nikel-contaning superoxide dismutase (NiSOD) has been isolated f ...
Second Year - WordPress.com
... a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Atomic number of any one element was found to be approximately the mea ...
... a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Atomic number of any one element was found to be approximately the mea ...
Theories of the constitution of gases in the early nineteenth century
... A hint that gases might owe their lightness to electricity was apparent in the experiments of Lavoisier and Laplace who generated gases on the top plate of an electroscope and observed the accumulation of an electric charge. They assumed that the gas must have escaped with the opposite charge.7 Boil ...
... A hint that gases might owe their lightness to electricity was apparent in the experiments of Lavoisier and Laplace who generated gases on the top plate of an electroscope and observed the accumulation of an electric charge. They assumed that the gas must have escaped with the opposite charge.7 Boil ...
effect of inorganic ions on the oxidation of dichlorvos insecticide with
... because ferrous ions are being consumed more rapidly than they are being produced. Current studies done on the Fenton method present fairly good results when applying the Fenton‘s reagent on toxic chemical and dyed waste water treatment[4]. ...
... because ferrous ions are being consumed more rapidly than they are being produced. Current studies done on the Fenton method present fairly good results when applying the Fenton‘s reagent on toxic chemical and dyed waste water treatment[4]. ...
Chemistry of CHLORINE
... c) (i) Why is the brown solid collected at the point as shown in method I and II. -Heated iron (III) Chloride crystals sublime to gas and solidify on the cooler parts. (ii) Name another metal that can be used in place of iron to react with chlorine and collected at similar point on heating explain. ...
... c) (i) Why is the brown solid collected at the point as shown in method I and II. -Heated iron (III) Chloride crystals sublime to gas and solidify on the cooler parts. (ii) Name another metal that can be used in place of iron to react with chlorine and collected at similar point on heating explain. ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.