Balancing Reaction Equations Oxidation State Reduction
... spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge. ...
... spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge. ...
Appendices - Mattson Creighton
... 1. A precipitate is an insoluble solid substance that is formed from an aqueous solution. Usually, precipitates are noticed as a cloudiness in the solution or as suspended particles. Eventually they settle to the bottom. 2. Limewater is a saturated solution of calcium hydroxide, Ca(OH)2(aq) 3. Carbo ...
... 1. A precipitate is an insoluble solid substance that is formed from an aqueous solution. Usually, precipitates are noticed as a cloudiness in the solution or as suspended particles. Eventually they settle to the bottom. 2. Limewater is a saturated solution of calcium hydroxide, Ca(OH)2(aq) 3. Carbo ...
1 - New Age International
... 9. Empirical formula: It is the relative number of different types of atoms present in a given compound. It is obtained from the analytical chemical data on the compound and the atomic masses of various elements present in the compound. 10. Molecular formula: It gives the exact number of atoms in a ...
... 9. Empirical formula: It is the relative number of different types of atoms present in a given compound. It is obtained from the analytical chemical data on the compound and the atomic masses of various elements present in the compound. 10. Molecular formula: It gives the exact number of atoms in a ...
carbon compounds - Badhan Education
... Carbon compounds large number compounds with hydrogen called hydrocarbons. Carbon can form compounds with hydrogen, oxygen, nitrogen, sulphur etc. Today, million of carbon compounds are known. These compounds are used in our daily life and they have brought lot of changes in our life. They are being ...
... Carbon compounds large number compounds with hydrogen called hydrocarbons. Carbon can form compounds with hydrogen, oxygen, nitrogen, sulphur etc. Today, million of carbon compounds are known. These compounds are used in our daily life and they have brought lot of changes in our life. They are being ...
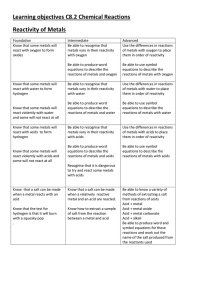
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... Use the differences in reactions of metals with oxygen to place them in order of reactivity ...
... Use the differences in reactions of metals with oxygen to place them in order of reactivity ...
Chemistry Test Ch 11 Stoichiometry
... 1. Balance the following equation and work the problems below: ____N2 + ___ H2 ___ NH3 A. If 6.52 L of H2 react with excess nitrogen what volume of NH3 is produced? B. In order to produce 6.52 L of NH3 how many liters of nitrogen are needed? C. If 2.35 x 1024 molecules of NH3 is formed how many gr ...
... 1. Balance the following equation and work the problems below: ____N2 + ___ H2 ___ NH3 A. If 6.52 L of H2 react with excess nitrogen what volume of NH3 is produced? B. In order to produce 6.52 L of NH3 how many liters of nitrogen are needed? C. If 2.35 x 1024 molecules of NH3 is formed how many gr ...
Endothermic reactions
... You have seen many reactions that release energy. Chemical reactions that release energy are called exergonic (ek sur GAH nihk) reactions. In these reactions, less energy is required to break the original bonds than is released when new bonds are formed. As a result, some form of energy, such as lig ...
... You have seen many reactions that release energy. Chemical reactions that release energy are called exergonic (ek sur GAH nihk) reactions. In these reactions, less energy is required to break the original bonds than is released when new bonds are formed. As a result, some form of energy, such as lig ...
Subject Area Assessment Guides
... or inter-atomic forces. When enough energy is added to the solid, the kinetic energy of the atoms and molecules increases sufficiently to overcome the attractive forces between the particles, and they break free of their fixed lattice positions. This ...
... or inter-atomic forces. When enough energy is added to the solid, the kinetic energy of the atoms and molecules increases sufficiently to overcome the attractive forces between the particles, and they break free of their fixed lattice positions. This ...
Stoichiometry - Norbraten
... Using the mole ratio we can get mass relations (the mole ratio cannot be directly applied to masses, i.e. there is technically no mass ratio!) ...
... Using the mole ratio we can get mass relations (the mole ratio cannot be directly applied to masses, i.e. there is technically no mass ratio!) ...
Oxidation numbers
... In fact, oxidation never takes place on its own - nor does reduction. When one substance is oxidised in a reaction, another one is reduced. A Redox reaction is one in which both reduction and oxidation take place. To work out which element is oxidised and which is reduced in a reaction, we go throug ...
... In fact, oxidation never takes place on its own - nor does reduction. When one substance is oxidised in a reaction, another one is reduced. A Redox reaction is one in which both reduction and oxidation take place. To work out which element is oxidised and which is reduced in a reaction, we go throug ...
File
... What is percentage yield and why do we use it? In chemical reactions we rarely, if ever, get the amount/quantity of products we calculate from a (balanced) chemical equation. The reasons for this can be: • at the end of the reaction there may be reactant left unconverted to product (see excess) • ...
... What is percentage yield and why do we use it? In chemical reactions we rarely, if ever, get the amount/quantity of products we calculate from a (balanced) chemical equation. The reasons for this can be: • at the end of the reaction there may be reactant left unconverted to product (see excess) • ...
Regents Review Questions
... (1) The equation represents a physical change, with the product and reactants having different chemical properties. (2) The equation represents a physical change, with the product and reactants having identical chemical properties. (3) The equation represents a chemical change, with the product and ...
... (1) The equation represents a physical change, with the product and reactants having different chemical properties. (2) The equation represents a physical change, with the product and reactants having identical chemical properties. (3) The equation represents a chemical change, with the product and ...
Biodiesel preparation in batch emulgation reactor
... The content of glycerides was determined in the verification experiment and fills the norm – monoglycerides: 0.39 wt-%; diglycerides: 0.17 wt-% and triglycerides 0.09 wt-% (determined by GC method according to EN 14105 at Shimadzu GC-2010). 4.3 Analysis of results Models for density (Y 2 ), acid nu ...
... The content of glycerides was determined in the verification experiment and fills the norm – monoglycerides: 0.39 wt-%; diglycerides: 0.17 wt-% and triglycerides 0.09 wt-% (determined by GC method according to EN 14105 at Shimadzu GC-2010). 4.3 Analysis of results Models for density (Y 2 ), acid nu ...
9.1 REDOX Introduction to Oxidation and Reduction
... Cr2O72- (aq) + Cl1- (aq) Cr 3+ (aq) + Cl2 (g) ...
... Cr2O72- (aq) + Cl1- (aq) Cr 3+ (aq) + Cl2 (g) ...
www.xtremepapers.net
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
Oxidation-Reduction Reactions
... reduced." There is no net change in the number of electrons in a redox reaction. Those given off in the oxidation half reaction are taken on by another species in the reduction half reaction. The two species that exchange electrons in a redox reaction are given special names. The ion or molecule th ...
... reduced." There is no net change in the number of electrons in a redox reaction. Those given off in the oxidation half reaction are taken on by another species in the reduction half reaction. The two species that exchange electrons in a redox reaction are given special names. The ion or molecule th ...
Fundamentals
... known that atoms are composed of yet smaller particles, an atom remains the smallest entity that an element can be broken down into and retain the properties of that element. Atoms can be imaged with two types of instruments: a scanning tunneling microscope (STM) and an atomic force microscope (AFM) ...
... known that atoms are composed of yet smaller particles, an atom remains the smallest entity that an element can be broken down into and retain the properties of that element. Atoms can be imaged with two types of instruments: a scanning tunneling microscope (STM) and an atomic force microscope (AFM) ...
SolarEnergy_Kit#1 - Institute for School Partnership
... requirements of humanity, maximizing its efficiency as a renewable energy source is essential. At this time, we use a significant fraction of this energy but we could learn to use it even more effectively in the future. Background: The process of photosynthesis involves the use of light energy to co ...
... requirements of humanity, maximizing its efficiency as a renewable energy source is essential. At this time, we use a significant fraction of this energy but we could learn to use it even more effectively in the future. Background: The process of photosynthesis involves the use of light energy to co ...
Chapter 8
... heat near the surface of the earth in the same manner in which a greenhouse is warmed. 45. The effects of global warming can be reduced by: 1. developing new energy sources (not dependant on fossil fuels) 2. conservation of energy resources 3. recycling 4. decreased destruction of the rain forests a ...
... heat near the surface of the earth in the same manner in which a greenhouse is warmed. 45. The effects of global warming can be reduced by: 1. developing new energy sources (not dependant on fossil fuels) 2. conservation of energy resources 3. recycling 4. decreased destruction of the rain forests a ...
AP CHEMISTRY – Source: 1999 AP Exam CHAPTER 8 PRACTICE
... 1. The energy required to convert a ground-state atom in the gas phase to a gaseous positive ion: C 2. The energy change that occurs in the conversion of an ionic solid to widely separated gaseous ions: E 3. The energy in a chemical or physical change that is available to do useful work: B 4. The en ...
... 1. The energy required to convert a ground-state atom in the gas phase to a gaseous positive ion: C 2. The energy change that occurs in the conversion of an ionic solid to widely separated gaseous ions: E 3. The energy in a chemical or physical change that is available to do useful work: B 4. The en ...
chemical reactions and energy changes
... but of a special kind. Substances such as these, where virtually all the material that dissolves breaks down into ions, are known as strong electrolytes. Most of the electrolytes you have met so far have been of the strong variety. This is true, for example, of all salts, substances that result from ...
... but of a special kind. Substances such as these, where virtually all the material that dissolves breaks down into ions, are known as strong electrolytes. Most of the electrolytes you have met so far have been of the strong variety. This is true, for example, of all salts, substances that result from ...
Unit F335/01
... (c) At 500 K, the equilibrium constant for equation 1.1 is 7.76 × 10–3. In an equilibrium mixture at 500 K, the concentrations of hydrogen and carbon dioxide are: [H2] = 1.00 × 10–5 mol dm–3 [CO2] = 3.46 × 10–5 mol dm–3 Calculate the equilibrium concentrations of H2O and CO at 500 K. Assume the H2O ...
... (c) At 500 K, the equilibrium constant for equation 1.1 is 7.76 × 10–3. In an equilibrium mixture at 500 K, the concentrations of hydrogen and carbon dioxide are: [H2] = 1.00 × 10–5 mol dm–3 [CO2] = 3.46 × 10–5 mol dm–3 Calculate the equilibrium concentrations of H2O and CO at 500 K. Assume the H2O ...
CIS Exam Questions
... A Increases the rate of the forward reaction only B Increases the rate of the reverse reaction only C Increases the rate of both the forward and reverse reactions D Changes the position of the equilibrium of the reaction 2. In which of the following systems will the equilibrium be unaffected by a ch ...
... A Increases the rate of the forward reaction only B Increases the rate of the reverse reaction only C Increases the rate of both the forward and reverse reactions D Changes the position of the equilibrium of the reaction 2. In which of the following systems will the equilibrium be unaffected by a ch ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.