Descriptive Chemistry of Elements d-Block
... coloured compounds. Normally, Sc and Zn are not considered as transition elements as Sc forms only Sc3+ with no d-electrons (3d0) whilst Zn forms only Zn2+ with a full d-sub shell (3d10). In some text books, Sc is considered as a transition metal because Zero valent scandium (Sc0) has a partly fille ...
... coloured compounds. Normally, Sc and Zn are not considered as transition elements as Sc forms only Sc3+ with no d-electrons (3d0) whilst Zn forms only Zn2+ with a full d-sub shell (3d10). In some text books, Sc is considered as a transition metal because Zero valent scandium (Sc0) has a partly fille ...
Standard - Santee Education Complex
... Metals are malleable, ductile, and have luster; most of the elements on the periodic table are metals. They oxidize (rust and tarnish) readily and form positive ions (cations). They are excellent conductors of both heat and electricity. The metals can be broken down into several groups. Transition m ...
... Metals are malleable, ductile, and have luster; most of the elements on the periodic table are metals. They oxidize (rust and tarnish) readily and form positive ions (cations). They are excellent conductors of both heat and electricity. The metals can be broken down into several groups. Transition m ...
Alkanes Chapter 1.1
... Branched Alkanes • A substituent group is an atom or group of atoms that replaces a hydrogen atom in an organic compound • An alkyl group is a type of substituent group made up of one or more carbon atoms • Branches are named using a three part prefix 1. A number to indicate which carbon on the mai ...
... Branched Alkanes • A substituent group is an atom or group of atoms that replaces a hydrogen atom in an organic compound • An alkyl group is a type of substituent group made up of one or more carbon atoms • Branches are named using a three part prefix 1. A number to indicate which carbon on the mai ...
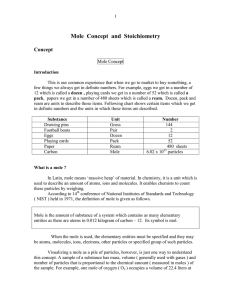
Mole Concept and Stoichiometry
... an amount of substance containing Avogadro’s number of particles. Avogadro’s number is equal to 6.02214199 x 1023 . Unlike pair, dozen and gross, the exact number of particles in a mole can not be counted. There are several reasons for this. First, the particles are too small and can not be seen eve ...
... an amount of substance containing Avogadro’s number of particles. Avogadro’s number is equal to 6.02214199 x 1023 . Unlike pair, dozen and gross, the exact number of particles in a mole can not be counted. There are several reasons for this. First, the particles are too small and can not be seen eve ...
Briefing Session on 2012 HKDSE Examination (December 2012)
... treatment process, state its purpose, and explain the ...
... treatment process, state its purpose, and explain the ...
File
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
Synergistic Effects of Pulse Light Emitting Diode on Growth
... 3. Results and Discussions 3.1 Effect of pulsed LED light on Nannochloropsis sp. growth In order to understand how pulsed LED could affect the growth of Nannochloropsis sp., two other light condition were used; i) red (660 nm), ii) blue. The pulsed LED light exhibits maximum cell concentration of Na ...
... 3. Results and Discussions 3.1 Effect of pulsed LED light on Nannochloropsis sp. growth In order to understand how pulsed LED could affect the growth of Nannochloropsis sp., two other light condition were used; i) red (660 nm), ii) blue. The pulsed LED light exhibits maximum cell concentration of Na ...
Week 7 - Acid-base, redox
... Cu(s) was oxidized because its oxidation number increased (or it lost electrons). Ag+ was reduced because its oxidation number decreased (or it gained electrons). When we use the term agent, such as oxidizing agent or reducing agent, it means the effect one chemical has on the other. As a result, co ...
... Cu(s) was oxidized because its oxidation number increased (or it lost electrons). Ag+ was reduced because its oxidation number decreased (or it gained electrons). When we use the term agent, such as oxidizing agent or reducing agent, it means the effect one chemical has on the other. As a result, co ...
Experimental skills and abilities
... 1 The evaporation process should be done very slowly. This is because sugar can easily char as it solidifies around the sides of the evaporating basin during the evaporating process. Also the crystallisation will require a lot longer for crystals to form from the concentrated solution and may need ...
... 1 The evaporation process should be done very slowly. This is because sugar can easily char as it solidifies around the sides of the evaporating basin during the evaporating process. Also the crystallisation will require a lot longer for crystals to form from the concentrated solution and may need ...
Module 9 Methods for Structure Determination Lecture 24 UV
... unstable compound (CH5+) is a powerful acid, and can protonate just about any other molecule. When it protonates our sample, a proton has been added rather than an electron removed, so the resulting particles are simple cations, not radical cations, and are generally more stable than the radical cat ...
... unstable compound (CH5+) is a powerful acid, and can protonate just about any other molecule. When it protonates our sample, a proton has been added rather than an electron removed, so the resulting particles are simple cations, not radical cations, and are generally more stable than the radical cat ...
Ch. 11-12 Supplements
... hydroxide, Al(OH)3 forming liquid water and aqueous aluminum sulfate, Al2(SO4)3. If 30.0 grams of sulfuric acid and 25.0 grams of aluminum hydroxide react… b. How many grams of each product will be formed? c. How many grams of excess reactant remain? 4) a. Write the balanced equation for lithium met ...
... hydroxide, Al(OH)3 forming liquid water and aqueous aluminum sulfate, Al2(SO4)3. If 30.0 grams of sulfuric acid and 25.0 grams of aluminum hydroxide react… b. How many grams of each product will be formed? c. How many grams of excess reactant remain? 4) a. Write the balanced equation for lithium met ...
Document
... 5. Halogens: The oxidation number of fluorine is -1. Each of the other halogens (Cl, Br, I) has an oxidation number of -1 in binary compounds, except when the other element is another halogen above it in the periodic table or the other element is oxygen. 6. Compounds and ions: The sum of the oxidat ...
... 5. Halogens: The oxidation number of fluorine is -1. Each of the other halogens (Cl, Br, I) has an oxidation number of -1 in binary compounds, except when the other element is another halogen above it in the periodic table or the other element is oxygen. 6. Compounds and ions: The sum of the oxidat ...
Direct Energy Conversion: Fuel Cells
... Solid oxide fuel cells (SOFC) can also utilize carbon monoxide (CO). This makes them more fuel flexible and also generally more efficient with available fuels, such as natural gas or propane. Hydrogen and CO can be produced from natural gas and other fuels by steam reforming, for example. Fuel cells ...
... Solid oxide fuel cells (SOFC) can also utilize carbon monoxide (CO). This makes them more fuel flexible and also generally more efficient with available fuels, such as natural gas or propane. Hydrogen and CO can be produced from natural gas and other fuels by steam reforming, for example. Fuel cells ...
to view or the PHOTOSYNTHESIS Presentation
... Scientists write equations to show chemical reactions The formation of water from hydrogen and oxygen is written as: ...
... Scientists write equations to show chemical reactions The formation of water from hydrogen and oxygen is written as: ...
summer fun - West Windsor-Plainsboro Regional School District
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
The Free High School Science Texts: A Textbook for High School
... Aside: Probabilities describe the chance of something happening or of being true. They usually have a value between 0 and 1 or 0% and 100% where 0 means no chance at all and 1 means definite. Probabilities are used when the state of something is uncertain. For example, probabilities are often used ...
... Aside: Probabilities describe the chance of something happening or of being true. They usually have a value between 0 and 1 or 0% and 100% where 0 means no chance at all and 1 means definite. Probabilities are used when the state of something is uncertain. For example, probabilities are often used ...
chemistry
... Base your answers to questions 59 through 61 on the information below. Carbon forms molecular compounds with some elements from Group 16. Two of these compounds are carbon dioxide, CO2, and carbon disulfide, CS2. Carbon dioxide is a colorless, odorless gas at room temperature. At standard temperatur ...
... Base your answers to questions 59 through 61 on the information below. Carbon forms molecular compounds with some elements from Group 16. Two of these compounds are carbon dioxide, CO2, and carbon disulfide, CS2. Carbon dioxide is a colorless, odorless gas at room temperature. At standard temperatur ...
Writing Chemical Reactions
... 10. complex ion formation or decomposition 11. Lewis acid/base (adduct formation) In the past these have occurred in any combination on an exam. In this course, we will break up these reactions into 7 groups and introduce a new group on each test. ____________________________________________________ ...
... 10. complex ion formation or decomposition 11. Lewis acid/base (adduct formation) In the past these have occurred in any combination on an exam. In this course, we will break up these reactions into 7 groups and introduce a new group on each test. ____________________________________________________ ...
A.P. Chemistry Writing Chemical Reactions Generally students do
... 10. complex ion formation or decomposition 11. Lewis acid/base (adduct formation) In the past these have occurred in any combination on an exam. In this course, we will break up these reactions into 7 groups and introduce a new group on each test. ____________________________________________________ ...
... 10. complex ion formation or decomposition 11. Lewis acid/base (adduct formation) In the past these have occurred in any combination on an exam. In this course, we will break up these reactions into 7 groups and introduce a new group on each test. ____________________________________________________ ...
Carbon Chemistry - North Allegheny School District
... the basis of life on Earth. A carbon atom has four electrons in its outer energy level, so it can form four covalent bonds with as many as four other atoms. When carbon atoms form four covalent bonds, they obtain the stability of a noble gas with eight electrons in their outer energy level. One of c ...
... the basis of life on Earth. A carbon atom has four electrons in its outer energy level, so it can form four covalent bonds with as many as four other atoms. When carbon atoms form four covalent bonds, they obtain the stability of a noble gas with eight electrons in their outer energy level. One of c ...
Moles Review
... 12) What is the empirical formula for the following molecular compounds: H2O2, C6H12O6, C6H6, CH4? 13) Given a compound that is composed of 0.6884 g of lead (Pb) , and 0.2356g chlorine (Cl), what is the empirical formula for this compound? 14) The most common form of nylon is 63.68% carbon, 12.38% n ...
... 12) What is the empirical formula for the following molecular compounds: H2O2, C6H12O6, C6H6, CH4? 13) Given a compound that is composed of 0.6884 g of lead (Pb) , and 0.2356g chlorine (Cl), what is the empirical formula for this compound? 14) The most common form of nylon is 63.68% carbon, 12.38% n ...
1 of 52
... below. In this equation, the · 8 H2O in Ba(OH)2 · 8 H2O indicates the presence of eight water molecules. This compound is called barium hydroxide octahydrate. (a) Balance the equation. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.) ...
... below. In this equation, the · 8 H2O in Ba(OH)2 · 8 H2O indicates the presence of eight water molecules. This compound is called barium hydroxide octahydrate. (a) Balance the equation. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.) ...
Electrochemistry
... circuit must be accompanied by a compensating transport of ions between the two cells. This means that we must provide a path for ions to move directly from one cell to the other. This ionic transport involves not only the electroactive species Cu2+ and Zn2+, but also the counterions, which in this ...
... circuit must be accompanied by a compensating transport of ions between the two cells. This means that we must provide a path for ions to move directly from one cell to the other. This ionic transport involves not only the electroactive species Cu2+ and Zn2+, but also the counterions, which in this ...
Cyanide Destruction with Chlorine Dioxide
... makes chlorine dioxide a powerful nonchlorinating oxidizing agent useful in many water treating applications for which chlorine and other oxidizing agents are unsuitable. Unlike most oxidants, chlorine dioxide may be used over a broad pH range. Application Description Cyanides (CN-) are used to solu ...
... makes chlorine dioxide a powerful nonchlorinating oxidizing agent useful in many water treating applications for which chlorine and other oxidizing agents are unsuitable. Unlike most oxidants, chlorine dioxide may be used over a broad pH range. Application Description Cyanides (CN-) are used to solu ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.