Document
... 700 W/m2 radient energy strikes a flat plate normally. The absorptivity is twice the transmitivity and 2.9 times its reflectivity. Determine the rate of absorption, transmission and reflection of energy in W/m2. ...
... 700 W/m2 radient energy strikes a flat plate normally. The absorptivity is twice the transmitivity and 2.9 times its reflectivity. Determine the rate of absorption, transmission and reflection of energy in W/m2. ...
Conductive Thermal Transfer
... He isotope ratio figure provided by Crossey et al. for the EarthScope 2010-2020 Science Plan… Black dots are travertines; color contour is P-velocity at 100 km depth. ...
... He isotope ratio figure provided by Crossey et al. for the EarthScope 2010-2020 Science Plan… Black dots are travertines; color contour is P-velocity at 100 km depth. ...
Ch 10 Review activity
... water have specific heats of 0.129 J/goC and 4.184 J/goC respectively, what is the final temperature of the mixture? Assume that the gold and the water experience no change in state of matter ...
... water have specific heats of 0.129 J/goC and 4.184 J/goC respectively, what is the final temperature of the mixture? Assume that the gold and the water experience no change in state of matter ...
heat engine
... An engine manufacturer makes the following claims: The heat input of the engine is 9 kW at 375 K. The heat output is 4 kW at 225 K. Do you believe these claims? ...
... An engine manufacturer makes the following claims: The heat input of the engine is 9 kW at 375 K. The heat output is 4 kW at 225 K. Do you believe these claims? ...
Atmospheric circulation
... Air moves from regions of high pressure to regions of low pressure Speed of fluid depends on the steepness of the ...
... Air moves from regions of high pressure to regions of low pressure Speed of fluid depends on the steepness of the ...
THERMODYNAMICS - FSU High Energy Physics
... between bodies of different temperature (i.e. of different average internal thermal energy), heat will flow from the body of higher temperature to the body of lower temperature until the temperatures of the two bodies are the same; then the bodies are in “thermal equilibrium” two bodies are in therm ...
... between bodies of different temperature (i.e. of different average internal thermal energy), heat will flow from the body of higher temperature to the body of lower temperature until the temperatures of the two bodies are the same; then the bodies are in “thermal equilibrium” two bodies are in therm ...
Spiral Store A PCM Thermal - Knowledge Transfer Ireland
... lightweight and is made up of a cylinder-shaped tank with a base. The tank contains layers, one to heat fluid, one to hold the PCM and one for secondary heated fluid. It is known as a “counter flow device”. The layers in the device work by using flow paths. Each layer has a first flow path and a sec ...
... lightweight and is made up of a cylinder-shaped tank with a base. The tank contains layers, one to heat fluid, one to hold the PCM and one for secondary heated fluid. It is known as a “counter flow device”. The layers in the device work by using flow paths. Each layer has a first flow path and a sec ...
ME 3210 Mechatronics – Thermal Systems
... consequence of this is that there are only first order differential equations to describe thermal systems. There may be multiple first order parts but never a second order, underdamped part. Thermal Capacitors An example of a thermal capacitor is a mass with a specific heat. When a mass has a temper ...
... consequence of this is that there are only first order differential equations to describe thermal systems. There may be multiple first order parts but never a second order, underdamped part. Thermal Capacitors An example of a thermal capacitor is a mass with a specific heat. When a mass has a temper ...
Review of 17.1, 17.2 and 17.3 Name: 1.) When 2 moles of NO burn
... Calculate the total energy change for converting the snow to hot water. qstep1= mc ∆t = 2390 x 2.01 J/g0C x 12.40C = 5.96 x 104 J ∆Hfus step2 = nHfus = (2390 /18.02)(6.03kJ/mol) = 8.00 x 10 5 J qstep3 = mc ∆t = 2390 x 4.19 J/g0C x 97.80C = 9.79 x 105 J TOTAL = 1.84 x 106 J = 1.84 x 103 kJ 5. Nuclear ...
... Calculate the total energy change for converting the snow to hot water. qstep1= mc ∆t = 2390 x 2.01 J/g0C x 12.40C = 5.96 x 104 J ∆Hfus step2 = nHfus = (2390 /18.02)(6.03kJ/mol) = 8.00 x 10 5 J qstep3 = mc ∆t = 2390 x 4.19 J/g0C x 97.80C = 9.79 x 105 J TOTAL = 1.84 x 106 J = 1.84 x 103 kJ 5. Nuclear ...
Heat Standard 4a/4d p. 400-409 1. The earth receives energy from
... waves, including water, light and sound waves, or by moving objects. ...
... waves, including water, light and sound waves, or by moving objects. ...
Homework #1: Energy Unit Conversions
... 4. 100.0 grams of 4.0°C water are heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy in Joules needed to cause this rise in temperature. ...
... 4. 100.0 grams of 4.0°C water are heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy in Joules needed to cause this rise in temperature. ...
Silicone Heat Transfer Compound
... Silicone Heat Transfer Compound is a metal oxide filled silicone oil providing an extremely efficient and exceptionally thermally conductive compound which will operate over a wide temperature range. Electrolube Heat Transfer Compound is recommended where the efficient and reliable thermal coupling ...
... Silicone Heat Transfer Compound is a metal oxide filled silicone oil providing an extremely efficient and exceptionally thermally conductive compound which will operate over a wide temperature range. Electrolube Heat Transfer Compound is recommended where the efficient and reliable thermal coupling ...
L 17
... • Refrigerators require energy input (work) (electricity) to operate. • Heat does not flow spontaneously from cold to hot, but it can be made to flow backwards if there is an input of WORK. • It uses electrical energy to pump heat from cold to hot. ...
... • Refrigerators require energy input (work) (electricity) to operate. • Heat does not flow spontaneously from cold to hot, but it can be made to flow backwards if there is an input of WORK. • It uses electrical energy to pump heat from cold to hot. ...
performance analysis of solar flat plate collector
... collectors (Parabolic trough, Fresnel etc.) may also be used, but since a large part of the annual irradiation is diffuse – especially in the northern part Europe – and of these types do not utilize the diffuse part, they are not described further in this fact sheet. ...
... collectors (Parabolic trough, Fresnel etc.) may also be used, but since a large part of the annual irradiation is diffuse – especially in the northern part Europe – and of these types do not utilize the diffuse part, they are not described further in this fact sheet. ...
Specific Heat and Calculating Heat Absorbed - Varga
... mass and the amount of heat required to raise a substance to a specific temperature. The symbol for mass is m. In other words, the relationship between mass and heat required is linear. ...
... mass and the amount of heat required to raise a substance to a specific temperature. The symbol for mass is m. In other words, the relationship between mass and heat required is linear. ...
Chem 1010 Tutorials Tutorial 9A – Heat and Work Fall 2013
... 105 kJ of work is performed on a system during compression as it releases 625 kJ of heat. What is the change in internal energy of the system? ...
... 105 kJ of work is performed on a system during compression as it releases 625 kJ of heat. What is the change in internal energy of the system? ...
Influence of supercritical ORC parameters on plate heat
... Low Tmax gradient U-p is larger influence of pmax is bigger For cte pmax and Tmax ↑ U↓ During superheating HTC between heat transfer fluid and vapour of working fluid is very low (superheating↑ U↓) o Influence of Tmax and pmax on A Influence of U Also to keep pinch point difference ...
... Low Tmax gradient U-p is larger influence of pmax is bigger For cte pmax and Tmax ↑ U↓ During superheating HTC between heat transfer fluid and vapour of working fluid is very low (superheating↑ U↓) o Influence of Tmax and pmax on A Influence of U Also to keep pinch point difference ...
Exercises - Madison County Schools
... 44. Explain why Europe is much warmer than northeastern Canada, even though they are at similar latitudes. ...
... 44. Explain why Europe is much warmer than northeastern Canada, even though they are at similar latitudes. ...
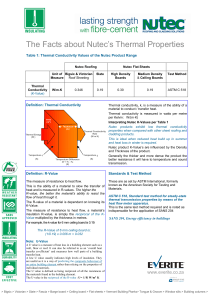
The Facts about Nutec Thermal Properties 11 5 12.pub
... The measure of resistance to heat flow. This is the ability of a material to slow the transfer of heat and is measured in R-values. The higher the R-value, the better the material's ability to resist the flow of heat through it. The R-value of a material is dependant on knowing its K-Value. The meas ...
... The measure of resistance to heat flow. This is the ability of a material to slow the transfer of heat and is measured in R-values. The higher the R-value, the better the material's ability to resist the flow of heat through it. The R-value of a material is dependant on knowing its K-Value. The meas ...
Temperature, Heat, and Expansion
... by falling masses turn in the water. The agitation warms the water and increases its internal energy. The temperature of the water is then measured, giving and indication of the water’s internal energy increase. If a total mass of 11.5 kg falls 1.3 m and all of the mechanical energy is converted to ...
... by falling masses turn in the water. The agitation warms the water and increases its internal energy. The temperature of the water is then measured, giving and indication of the water’s internal energy increase. If a total mass of 11.5 kg falls 1.3 m and all of the mechanical energy is converted to ...
calorimetry - Saddleback College
... will happen when you mix two samples which are initially at different temperatures. If your prediction is correct, then you can feel somewhat confident in your assumptions, until they lead to a prediction that turns out to be wrong. Assumption One: (Zeroth Law of Thermodynamics): Assume that if two ...
... will happen when you mix two samples which are initially at different temperatures. If your prediction is correct, then you can feel somewhat confident in your assumptions, until they lead to a prediction that turns out to be wrong. Assumption One: (Zeroth Law of Thermodynamics): Assume that if two ...
floor level coverage charts
... FLOOR LEVEL COVERAGE CHARTS The important factors in designing an infrared heating system are: Satisfying the building heat loss (the most important factor in achieving the desired inside design temperature). For detailed information, refer to the Application Section, Section IV, Heat Loss Calcula ...
... FLOOR LEVEL COVERAGE CHARTS The important factors in designing an infrared heating system are: Satisfying the building heat loss (the most important factor in achieving the desired inside design temperature). For detailed information, refer to the Application Section, Section IV, Heat Loss Calcula ...
Thermal Energy - St. Thomas the Apostle School
... What types of heating systems are used in your home? ...
... What types of heating systems are used in your home? ...
introduction - IIT Portal.com
... Its knowledge should also be imported for efficient working of various machines. Calorimetry and Thermas Expansion:Calorimetry:Heat:It is a form of energy which determines the change in thermas state of a body. Heat flows from a body which has a higher temp. to the body which has lower temp. Specifi ...
... Its knowledge should also be imported for efficient working of various machines. Calorimetry and Thermas Expansion:Calorimetry:Heat:It is a form of energy which determines the change in thermas state of a body. Heat flows from a body which has a higher temp. to the body which has lower temp. Specifi ...
Solar water heating

Solar water heating (SWH) is the conversion of sunlight into renewable energy for water heating using a solar thermal collector. Solar water heating systems comprise various technologies that are used worldwide increasingly.In a ""close-coupled"" SWH system the storage tank is horizontally mounted immediately above the solar collectors on the roof. No pumping is required as the hot water naturally rises into the tank through thermosiphon flow. In a ""pump-circulated"" system the storage tank is ground- or floor-mounted and is below the level of the collectors; a circulating pump moves water or heat transfer fluid between the tank and the collectors.SWH systems are designed to deliver hot water for most of the year. However, in winter there sometimes may not be sufficient solar heat gain to deliver sufficient hot water. In this case a gas or electric booster is used to heat the water.