Unit 5: Chemical Equations and Reactions
... Chemical. New subtance (ashes) is formed. The ashes cannot be turned back into the original plywood. d. alcohol is distilled from an alcohol-water solution Physical. The alcohol merely separated from the alcohol-water solution. The mixture can be reconstituted by mixing alcohol and water again. ...
... Chemical. New subtance (ashes) is formed. The ashes cannot be turned back into the original plywood. d. alcohol is distilled from an alcohol-water solution Physical. The alcohol merely separated from the alcohol-water solution. The mixture can be reconstituted by mixing alcohol and water again. ...
Encoded Digital Periodic Table
... cybernetic methods, information theory and system theory, respectively. This paper is to report that we discovered new methods for development of the new technologies in chemistry. It is about the most advanced digital technology which is based on program, cybernetics and informational systems and l ...
... cybernetic methods, information theory and system theory, respectively. This paper is to report that we discovered new methods for development of the new technologies in chemistry. It is about the most advanced digital technology which is based on program, cybernetics and informational systems and l ...
Chapter 6 Chemical Composition
... • One amu is one-twelfth of the mass of a 12C atom • One amu is close to the mass of one proton or one neutron. • One amu is a very small mass – 1.66 x 10-24 g • One mole is 6.022 x 1023 units of anything • One mole (of atoms) of an element will have a mass in grams equal to the mass in amu of one a ...
... • One amu is one-twelfth of the mass of a 12C atom • One amu is close to the mass of one proton or one neutron. • One amu is a very small mass – 1.66 x 10-24 g • One mole is 6.022 x 1023 units of anything • One mole (of atoms) of an element will have a mass in grams equal to the mass in amu of one a ...
PowerPoint Presentation - Formulas, Equations, and Moles
... One mole of a substance is the gram mass value equal to the amu mass of the substance. ...
... One mole of a substance is the gram mass value equal to the amu mass of the substance. ...
chemical reaction
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
Solid-State and High-Resolution Liquid 119Sn NMR Spectroscopy
... Table 2, the hybrid orbital containing the lone pair gains more s character. This gain in s character at tin translates into increased shielding owing to the increased electron density at the 119Sn nucleus. Thus the most shielded δ33 value of -165.1 ppm is observed for the most electronegative subst ...
... Table 2, the hybrid orbital containing the lone pair gains more s character. This gain in s character at tin translates into increased shielding owing to the increased electron density at the 119Sn nucleus. Thus the most shielded δ33 value of -165.1 ppm is observed for the most electronegative subst ...
mole
... hydrogen carbonate are reacted together. The products formed are aqueous sodium chloride, water, and carbon dioxide gas. Write a skeleton equation for this reaction. • Solid sodium hydrogen carbonate: NaHCO3(s) • Hydrochloric acid: HCl(aq) • Water: H2O(l) • Carbon dioxide gas: CO2(g) ...
... hydrogen carbonate are reacted together. The products formed are aqueous sodium chloride, water, and carbon dioxide gas. Write a skeleton equation for this reaction. • Solid sodium hydrogen carbonate: NaHCO3(s) • Hydrochloric acid: HCl(aq) • Water: H2O(l) • Carbon dioxide gas: CO2(g) ...
mc_ch08 - MrBrownsChem1LCHS
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
CBSE Living Science Chemistry Class X
... Showing the changes taking place when granulated zinc is added to dilute sulphuric acid Take 20 mL of dil. H2SO4 in a conical flask fitted with a cork and a delivery tube having a fine jet. Clamp the conical flask to the clamp stand (Fig. 1.2). Add some pieces of granulated zinc to the conical flask ...
... Showing the changes taking place when granulated zinc is added to dilute sulphuric acid Take 20 mL of dil. H2SO4 in a conical flask fitted with a cork and a delivery tube having a fine jet. Clamp the conical flask to the clamp stand (Fig. 1.2). Add some pieces of granulated zinc to the conical flask ...
1 - Grygla School
... Using reactions to manufacture chemicals is a big industry. Table 1 lists the top eight chemicals made in the United States. Some of these chemicals may be familiar, and some you may have never heard of. By the end of this course, you will know a lot more about them. Chemicals produced on a small sc ...
... Using reactions to manufacture chemicals is a big industry. Table 1 lists the top eight chemicals made in the United States. Some of these chemicals may be familiar, and some you may have never heard of. By the end of this course, you will know a lot more about them. Chemicals produced on a small sc ...
8 Chemical Equations Chapter Outline Chemical Equations
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
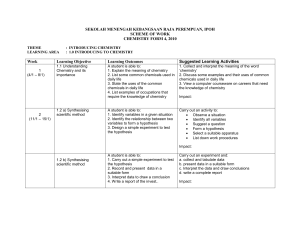
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... Gather information and discuss a. the similarities in chemical properties of lithium, sodium and potassium b. the relationship between the chemical properties of Group 1 elements and their electron arrangements Study the reaction of lithium, sodium and potassium with chlorine and bromine through com ...
... Gather information and discuss a. the similarities in chemical properties of lithium, sodium and potassium b. the relationship between the chemical properties of Group 1 elements and their electron arrangements Study the reaction of lithium, sodium and potassium with chlorine and bromine through com ...
In situ Raman Spectroscopic Study of Supported Molten Salt
... role in a number of industrial processes, which due to the associated sulfur oxide emissions have significant environmental impact. Although the main source of SO2 emissions to the atmosphere is the coal-fired power generation, large amounts of SO2 are also emitted from sulfuric acid manufacturers a ...
... role in a number of industrial processes, which due to the associated sulfur oxide emissions have significant environmental impact. Although the main source of SO2 emissions to the atmosphere is the coal-fired power generation, large amounts of SO2 are also emitted from sulfuric acid manufacturers a ...
Chemistry Unit Outcomes
... List the names of the first persons to recognize that it would be convenient to represent chemical substances using symbols? 2. Outline what John Dalton, an English chemist, did in 1808. 3. Explain how Dalton represented element and why there was a problem with Dalton’s system. 4. Define the term ch ...
... List the names of the first persons to recognize that it would be convenient to represent chemical substances using symbols? 2. Outline what John Dalton, an English chemist, did in 1808. 3. Explain how Dalton represented element and why there was a problem with Dalton’s system. 4. Define the term ch ...
Honors Chemistry I
... elemental iron when heated to extremely high temperature with either carbon monoxide or elemental hydrogen gas. Balanced the following equations for these two processes: a. Fe3O4(s) + H2(g) Fe(s) + H2O(g) b. Fe3O4(s) + CO(g) Fe(s) + CO2(g) 3) Chromium compounds exhibit a wide variety of bright c ...
... elemental iron when heated to extremely high temperature with either carbon monoxide or elemental hydrogen gas. Balanced the following equations for these two processes: a. Fe3O4(s) + H2(g) Fe(s) + H2O(g) b. Fe3O4(s) + CO(g) Fe(s) + CO2(g) 3) Chromium compounds exhibit a wide variety of bright c ...
Philosophy of Chemistry
... overcome received distinctions between science and technology. Studies on the formal sign language system of chemistry, consisting of structural formulas and reaction mechanisms, have illuminated its multi-purposed theoretical capacity; but further studies are required to understand its change due t ...
... overcome received distinctions between science and technology. Studies on the formal sign language system of chemistry, consisting of structural formulas and reaction mechanisms, have illuminated its multi-purposed theoretical capacity; but further studies are required to understand its change due t ...
Chapter 8 - profpaz.com
... Consequently, unless there are more ingredients, only 15 pancakes can be made from the combination of the above ingredients. This is due to the flour being completely used up after making 15 pancakes. ...
... Consequently, unless there are more ingredients, only 15 pancakes can be made from the combination of the above ingredients. This is due to the flour being completely used up after making 15 pancakes. ...
InChI keys as standard global identifiers in chemistry web services
... The objective of the IUPAC Chemical Identifier Project is to establish a unique label, the IUPAC Chemical Identifier, which would be a non-proprietary identifier for chemical substances that could be used in printed and electronic data sources thus enabling easier linking of diverse data compilation ...
... The objective of the IUPAC Chemical Identifier Project is to establish a unique label, the IUPAC Chemical Identifier, which would be a non-proprietary identifier for chemical substances that could be used in printed and electronic data sources thus enabling easier linking of diverse data compilation ...
CHEMISTRY SEC 06 SYLLABUS
... Knowledge of experimental details to determine melting point and boiling point are not required but pupils should be able to interpret a simple heating / cooling curve. It is suggested that examples are chosen from substances mentioned in Section 5.4(b). (e.g. sodium chloride, sodium carbonate and o ...
... Knowledge of experimental details to determine melting point and boiling point are not required but pupils should be able to interpret a simple heating / cooling curve. It is suggested that examples are chosen from substances mentioned in Section 5.4(b). (e.g. sodium chloride, sodium carbonate and o ...
Section 2 Types of Chemical Reactions
... • Two additional hydrogen atoms are needed on the right side of the equation. • Now increase the oxygen atoms by placing the coefficient 2 in front of the molecular formula for oxygen. The correct chemical equation, or balanced formula equation, for the burning of methane in oxygen is ...
... • Two additional hydrogen atoms are needed on the right side of the equation. • Now increase the oxygen atoms by placing the coefficient 2 in front of the molecular formula for oxygen. The correct chemical equation, or balanced formula equation, for the burning of methane in oxygen is ...
Infant formula
... 3.2.1 Milk-based infant formula: refers to liquid or powder products made only through physical methods, of which the main material is milk and milk protein products, supplemented with a proper amount of vitamin, minerals and other supplementary materials, which are applicable to normal infants, whe ...
... 3.2.1 Milk-based infant formula: refers to liquid or powder products made only through physical methods, of which the main material is milk and milk protein products, supplemented with a proper amount of vitamin, minerals and other supplementary materials, which are applicable to normal infants, whe ...
chemistry-c7-what-you-should
... I understand and use the terms ‘bulk’ (made on a large scale) and ‘fine’ (made on a small scale) in the context of the chemical industry I can recall examples of chemicals made on a large scale (ammonia, sulfuric acid, sodium hydroxide, phosphoric acid) and examples of chemicals made on a small scal ...
... I understand and use the terms ‘bulk’ (made on a large scale) and ‘fine’ (made on a small scale) in the context of the chemical industry I can recall examples of chemicals made on a large scale (ammonia, sulfuric acid, sodium hydroxide, phosphoric acid) and examples of chemicals made on a small scal ...
Naming Binary Molecular Compounds
... Note how in this example parenthesis surround a polyatomic anion and the subscript refers to the entire unit ...
... Note how in this example parenthesis surround a polyatomic anion and the subscript refers to the entire unit ...
08_Lecture - HCC Learning Web
... with water. These are called active metals. • The active metals are Li, Na, K, Rb, Cs, Ca, Sr, and Ba. • They react with water to produce a metal hydroxide and hydrogen gas. 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g) Ca(s) + 2 H2O(l) → Ca(OH)2(aq) + H2(g) © 2011 Pearson Education, Inc. ...
... with water. These are called active metals. • The active metals are Li, Na, K, Rb, Cs, Ca, Sr, and Ba. • They react with water to produce a metal hydroxide and hydrogen gas. 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g) Ca(s) + 2 H2O(l) → Ca(OH)2(aq) + H2(g) © 2011 Pearson Education, Inc. ...
Chemical industry

The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials (oil, natural gas, air, water, metals, and minerals) into more than 70,000 different products.