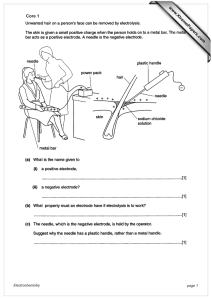
What is matter? - Waterford Public Schools
... • One can calculate the average atomic weight of an element if the abundance of each isotope for that element is known Average Atomic Mass % natural abundance ...
... • One can calculate the average atomic weight of an element if the abundance of each isotope for that element is known Average Atomic Mass % natural abundance ...
Periodic Law
... It was found that if Mendeleev's table was ordered by atomic number instead of atomic mass the inconsistencies in the table were eliminated. This is the blueprint for the modern periodic table. ...
... It was found that if Mendeleev's table was ordered by atomic number instead of atomic mass the inconsistencies in the table were eliminated. This is the blueprint for the modern periodic table. ...
Mendeleev`s periodic table
... elements not being whole numbers. HT ONLY (extending task): Calculate the relative atomic mass of an element from the relative masses and abundances of its isotopes. ...
... elements not being whole numbers. HT ONLY (extending task): Calculate the relative atomic mass of an element from the relative masses and abundances of its isotopes. ...
reviewTWO
... 6) Potassium bromide added to lithium iodide makes lithium bromide and potassium iodide. 7) When silver(I) nitrate is added to calcium chloride, calcium nitrate and silver(I) chloride are produced. 8) Potassium sulphate plus magnesium chloride forms magnesium sulphate and potassium chloride. 9) When ...
... 6) Potassium bromide added to lithium iodide makes lithium bromide and potassium iodide. 7) When silver(I) nitrate is added to calcium chloride, calcium nitrate and silver(I) chloride are produced. 8) Potassium sulphate plus magnesium chloride forms magnesium sulphate and potassium chloride. 9) When ...
Summer_Assignment_AP_Chemistry_TW 2015
... To the AP Chemistry Student: Welcome to my AP Chemistry class! I am looking forward to helping you gain a deeper appreciation for the science of chemistry and how it impacts our lives. I hope you are looking forward to an exciting and challenging year. Since you have elected to take this course, I a ...
... To the AP Chemistry Student: Welcome to my AP Chemistry class! I am looking forward to helping you gain a deeper appreciation for the science of chemistry and how it impacts our lives. I hope you are looking forward to an exciting and challenging year. Since you have elected to take this course, I a ...
Atomic Structure PPQs 2
... The graph below shows how the second ionisation energy of six consecutive elements in the Periodic Table, represented by the letters A to F, varies with increasing atomic ...
... The graph below shows how the second ionisation energy of six consecutive elements in the Periodic Table, represented by the letters A to F, varies with increasing atomic ...
2 periodic table cp
... to the loss or gain of electrons - If an atom loses elecs. it becomes smaller and positive - If an atom gains elecs. it becomes larger and negative ...
... to the loss or gain of electrons - If an atom loses elecs. it becomes smaller and positive - If an atom gains elecs. it becomes larger and negative ...
2 periodic table pd9
... d. noble gases = __ valence We are taking an ATB quiz on today’s ATBs in 5 minutes. ...
... d. noble gases = __ valence We are taking an ATB quiz on today’s ATBs in 5 minutes. ...
Chapter 5 Chem classnotes
... Ionization energy (IE) is the energy required to remove an electron from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. ...
... Ionization energy (IE) is the energy required to remove an electron from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. ...
Periodic table of elements
... All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
... All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
Atoms - Schoolwires.net
... measured the mass of a young willow tree and, separately, the mass of a bucket of soil and then planted the tree in the bucket. After five years, he found that the tree had gained 75 kg in mass even though the soil had lost only 0.057 kg. He had added only water to the bucket, and so he concluded th ...
... measured the mass of a young willow tree and, separately, the mass of a bucket of soil and then planted the tree in the bucket. After five years, he found that the tree had gained 75 kg in mass even though the soil had lost only 0.057 kg. He had added only water to the bucket, and so he concluded th ...
The placement of an element on the periodic table gives clues about
... all have the same number of valence electrons and therefore similar chemical properties. Valence electrons help in predicting chemical reactions. For example, since hydrogen (H) is in group 1, it has 1 electron in its outer energy level. Chlorine (Cl), which is in group 17, has 7 electrons in its ou ...
... all have the same number of valence electrons and therefore similar chemical properties. Valence electrons help in predicting chemical reactions. For example, since hydrogen (H) is in group 1, it has 1 electron in its outer energy level. Chlorine (Cl), which is in group 17, has 7 electrons in its ou ...
Chapter 12 Packet
... How many grams of HNO3 are needed to dissolve 11.45g of Cu? 21) The reaction of powdered aluminum and iron(II)oxide, 2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(l) produces so much heat the iron that forms is molten. Because of this, railroads use the reaction to provide molten steel to weld steel rails toge ...
... How many grams of HNO3 are needed to dissolve 11.45g of Cu? 21) The reaction of powdered aluminum and iron(II)oxide, 2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(l) produces so much heat the iron that forms is molten. Because of this, railroads use the reaction to provide molten steel to weld steel rails toge ...
Chemical Reactions
... Note the number of moles of gas on the left-hand side and the number of moles of gas on the righthand side. When the volume of the system is changed, the partial pressures of the gases change. If we were to decrease pressure by increasing volume, the equilibrium of the above reaction will shift to t ...
... Note the number of moles of gas on the left-hand side and the number of moles of gas on the righthand side. When the volume of the system is changed, the partial pressures of the gases change. If we were to decrease pressure by increasing volume, the equilibrium of the above reaction will shift to t ...
C1 Atomic Structure Grade Descriptor
... I can justify why the model of the atom has changed over time. I can evaluate the current model of an atom. I can use the periodic table to find atomic number and mass number data and use it to determine the number of each subatomic particle in any given atom. I can recognise and describe patterns i ...
... I can justify why the model of the atom has changed over time. I can evaluate the current model of an atom. I can use the periodic table to find atomic number and mass number data and use it to determine the number of each subatomic particle in any given atom. I can recognise and describe patterns i ...
C1 Self Assessment Checklist
... I can justify why the model of the atom has changed over time. I can evaluate the current model of an atom. I can use the periodic table to find atomic number and mass number data and use it to determine the number of each subatomic particle in any given atom. I can recognise and describe patterns i ...
... I can justify why the model of the atom has changed over time. I can evaluate the current model of an atom. I can use the periodic table to find atomic number and mass number data and use it to determine the number of each subatomic particle in any given atom. I can recognise and describe patterns i ...
Chapter 5 Organizing The Elements
... • Most reactive nonmetals are on the right side (group 17) • Copy fig. 13 into your notebooks • Complete questions 1-5 and 7 ...
... • Most reactive nonmetals are on the right side (group 17) • Copy fig. 13 into your notebooks • Complete questions 1-5 and 7 ...
Periodic Table
... He moved cards to positions where they _______________________________________ This left ___________________ blank spaces. Mendeleev proposed that the blank spaces would be _____________________________ _________________________________________________________. He even predicted their ______________ ...
... He moved cards to positions where they _______________________________________ This left ___________________ blank spaces. Mendeleev proposed that the blank spaces would be _____________________________ _________________________________________________________. He even predicted their ______________ ...
PROFESSIONAL LEARNING COMMUNITY MODEL FOR ENTRY
... with similar chemical or physical properties fall into vertical columns called groups or families. Prominent groups include alkali metals, alkaline earth metals, halogens, and noble gases. Regions of the periodic table are also broken up into two different regions: main group (or representative) ele ...
... with similar chemical or physical properties fall into vertical columns called groups or families. Prominent groups include alkali metals, alkaline earth metals, halogens, and noble gases. Regions of the periodic table are also broken up into two different regions: main group (or representative) ele ...
Study Guide
... Reactivity in nonmetals increases as atomic number decreases, so Fluorine is the most reactive nonmetal. Halogens react with alkali metals to form salts. Elements in the halogen family exist in all three phases. Fluorine (F) and Chlorine (Cl) are gases, Bromine (Br) is a liquid, and Iodine (I) and A ...
... Reactivity in nonmetals increases as atomic number decreases, so Fluorine is the most reactive nonmetal. Halogens react with alkali metals to form salts. Elements in the halogen family exist in all three phases. Fluorine (F) and Chlorine (Cl) are gases, Bromine (Br) is a liquid, and Iodine (I) and A ...
AQA Additional Sci C2 Revision Guide
... Alloys are usually made from two or more different metals. Alloying metals changes their properties and results in new materials which are more suited to their different uses. Most metals in everyday use are alloys e.g. pure gold is too soft to make jewellery but it can be hardened by adding metals ...
... Alloys are usually made from two or more different metals. Alloying metals changes their properties and results in new materials which are more suited to their different uses. Most metals in everyday use are alloys e.g. pure gold is too soft to make jewellery but it can be hardened by adding metals ...
Periodic Table How did Dmitri Mendeleev arrange the periodic table?
... • Each column of elements from top to bottom on the Periodic Table. • Also known as Family • Elements in a ‘family’ behave in a similar way – Example: Group 1 (all except Hydrogen) elements are called alkali metals. How are they similar? • They react explosively with water! ...
... • Each column of elements from top to bottom on the Periodic Table. • Also known as Family • Elements in a ‘family’ behave in a similar way – Example: Group 1 (all except Hydrogen) elements are called alkali metals. How are they similar? • They react explosively with water! ...