Opening Questions - Belle Vernon Area School District
... the carbon skeleton are covalent bonds known as PEPTIDE BONDS Many peptide bonds are called POLYPEPTIDE bonds ...
... the carbon skeleton are covalent bonds known as PEPTIDE BONDS Many peptide bonds are called POLYPEPTIDE bonds ...
Concept review: Chromatography (applied to protein purification)
... • 1. Cell disruption should be performed at cold temperatures. Keep the sample on ice as much as possible and use chilled solutions. This will decrease the activity of the proteases for the simple reasons that all chemical reactions occur more slowly at low temperature. • 2. Add protease inhibitors ...
... • 1. Cell disruption should be performed at cold temperatures. Keep the sample on ice as much as possible and use chilled solutions. This will decrease the activity of the proteases for the simple reasons that all chemical reactions occur more slowly at low temperature. • 2. Add protease inhibitors ...
Sample exam 1
... b. Identify the Roman numeral point at the isoelectric point. Draw a predominant structure or otherwise explain your choice. 7. The protein myoglobin is found in numerous organisms, and the amino acid residue sequence of the protein from a wide variety of organisms has been determined. Recall that ...
... b. Identify the Roman numeral point at the isoelectric point. Draw a predominant structure or otherwise explain your choice. 7. The protein myoglobin is found in numerous organisms, and the amino acid residue sequence of the protein from a wide variety of organisms has been determined. Recall that ...
Lecture2
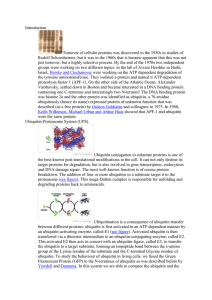
... the ubiquitin-proteasome pathway. Ubiquitin is highly conserved in all eukaryotes; it is a marker that targets proteins for rapid proteolysis. Ubiquitin is attached to the amino group of the side chain of a lysine residue, then more are added to form a chain. Polyubiquinated proteins are recognized ...
... the ubiquitin-proteasome pathway. Ubiquitin is highly conserved in all eukaryotes; it is a marker that targets proteins for rapid proteolysis. Ubiquitin is attached to the amino group of the side chain of a lysine residue, then more are added to form a chain. Polyubiquinated proteins are recognized ...
Dr. Elisar Barbar`s Lab - Oregon State University
... • Two classes of dynein – Axonemal dynein • propels beating of cilia and flagella – Cytoplasmic dynein • transports membrane bound vesicles, protein complexes, chromosomes • Cellular organization • Cell Division ...
... • Two classes of dynein – Axonemal dynein • propels beating of cilia and flagella – Cytoplasmic dynein • transports membrane bound vesicles, protein complexes, chromosomes • Cellular organization • Cell Division ...
File - Heritage FFA
... and also transports waste products away. Water is necessary for certain chemical reactions to occur. Water acts as the body's cooling system and helps regulate body heat. Water also acts as a lubricant for the body's organs. Any living thing can live longer without food than without water. PROTEINS ...
... and also transports waste products away. Water is necessary for certain chemical reactions to occur. Water acts as the body's cooling system and helps regulate body heat. Water also acts as a lubricant for the body's organs. Any living thing can live longer without food than without water. PROTEINS ...
Super ShieldTM HRP Conjugate Stabilizer
... capability to make it work for you. “With ImmunO4, it was easy to get formulations that worked in my assays.” ...
... capability to make it work for you. “With ImmunO4, it was easy to get formulations that worked in my assays.” ...
domain_searching.pdf
... 4. A list of the proteins in which this domain is found will come up Note the number of proteins in which this domain is found (in this case 75) in the table on the next page. 5. Below, list at least two different proteins that contain your protein in humans. The names of proteins are often written ...
... 4. A list of the proteins in which this domain is found will come up Note the number of proteins in which this domain is found (in this case 75) in the table on the next page. 5. Below, list at least two different proteins that contain your protein in humans. The names of proteins are often written ...
No Slide Title
... • Group of residues with high contact density, number of contacts within domains is higher than the number of contacts between domains. • A stable unit of protein structure that can fold autonomously • A rigid body linked to other domains by flexible linkers • A portion of the protein that can be ac ...
... • Group of residues with high contact density, number of contacts within domains is higher than the number of contacts between domains. • A stable unit of protein structure that can fold autonomously • A rigid body linked to other domains by flexible linkers • A portion of the protein that can be ac ...
Document
... number of potential serine/threonine amino acids that are potential phosphorylation sites. Based on this data, you hypothesize that this protein may be a substrate for a A. protein kinase. B. receptor tyrosine kinase. C. G-protein-coupled receptor. D. ADP-ribosylase. Why is phosphorylation-dephospho ...
... number of potential serine/threonine amino acids that are potential phosphorylation sites. Based on this data, you hypothesize that this protein may be a substrate for a A. protein kinase. B. receptor tyrosine kinase. C. G-protein-coupled receptor. D. ADP-ribosylase. Why is phosphorylation-dephospho ...
Photosynthesis in cyanobacteria and plants Simple Z Scheme for
... Plants and cyanobacteria use the reducing power generated by the light-driven oxidation of H2O to produce NADPH this is an uphill battle and photosynthesis therefore requires at least 810 photons of visible light to produce one molecule of oxygen Two processes are involved in photosynthesis (usually ...
... Plants and cyanobacteria use the reducing power generated by the light-driven oxidation of H2O to produce NADPH this is an uphill battle and photosynthesis therefore requires at least 810 photons of visible light to produce one molecule of oxygen Two processes are involved in photosynthesis (usually ...
RACK-1, a receptor for activated C kinase, links metabotropic
... Local protein synthesis is activated by glutamate in synaptoneurosomes (Weiler, Greenough PNAS, 90:7168, 1993). To search for transmitter receptor triggered mechanisms involved in translational control of dendritically localized mRNAs, we focussed on mRNPs that might be affected by second messenger ...
... Local protein synthesis is activated by glutamate in synaptoneurosomes (Weiler, Greenough PNAS, 90:7168, 1993). To search for transmitter receptor triggered mechanisms involved in translational control of dendritically localized mRNAs, we focussed on mRNPs that might be affected by second messenger ...
UTM EatWell Are Protein Powders Right For You?
... muscle, lose weight, or achieve some fitness goal, does not need a protein supplement or protein powder. The Coaching Association of Canada, Dietitians of Canada, the American College of Sports Medicine, and the American Dietetic Association all agree that “…protein recommendations for endurance and ...
... muscle, lose weight, or achieve some fitness goal, does not need a protein supplement or protein powder. The Coaching Association of Canada, Dietitians of Canada, the American College of Sports Medicine, and the American Dietetic Association all agree that “…protein recommendations for endurance and ...
Quality Control
... Proteins that form aggregated cellular inclusions ER proteins CFTR. delta-508 mutation is the most common cause of Cystic Fibrosis, and makes biogenesis of membrane protein even less efficient Immunoglobulins. Somatic hypermutation of Ig, especially visible in plasma cells alpha1 anti-trypsi ...
... Proteins that form aggregated cellular inclusions ER proteins CFTR. delta-508 mutation is the most common cause of Cystic Fibrosis, and makes biogenesis of membrane protein even less efficient Immunoglobulins. Somatic hypermutation of Ig, especially visible in plasma cells alpha1 anti-trypsi ...
Introduction
... Turnover of cellular proteins was discovered in the 1930s in studies of Rudolf Schoenheimer, but it was in the 1960s that is became apparent that this was not just turnover, but a highly selective process. By the end of the 1970s two independent groups were working on two different topics: in the la ...
... Turnover of cellular proteins was discovered in the 1930s in studies of Rudolf Schoenheimer, but it was in the 1960s that is became apparent that this was not just turnover, but a highly selective process. By the end of the 1970s two independent groups were working on two different topics: in the la ...
Protein levels with and without Monensin for
... Service. Contents of this publication may be freely reproduced for educational purposes. All other rights reserved. Brand names appearing in this publication are for product identification purposes only. K-State Research and Extension is an equal opportunity provider and employer. ...
... Service. Contents of this publication may be freely reproduced for educational purposes. All other rights reserved. Brand names appearing in this publication are for product identification purposes only. K-State Research and Extension is an equal opportunity provider and employer. ...
Abstracts
... After proteins are synthesized in the endoplasmic reticulum, they need to be transported to proper subcellular regions, such as nucleus, mitochondria, and cytoplasmic membranes. Proteins expressed on plasma membranes are known to be removed by a process called endocytosis, and enter recycling or deg ...
... After proteins are synthesized in the endoplasmic reticulum, they need to be transported to proper subcellular regions, such as nucleus, mitochondria, and cytoplasmic membranes. Proteins expressed on plasma membranes are known to be removed by a process called endocytosis, and enter recycling or deg ...
Magic Numbers in Protein Structures
... the action of the hydrophobic or hydrophillic force, which is an unspecific interface-tension-like force [1]. Yet a protein with a specific amino acid chain folds, paradoxically [3] in a matter of seconds, to a particular fold, according to information which must be provided via the underlying linea ...
... the action of the hydrophobic or hydrophillic force, which is an unspecific interface-tension-like force [1]. Yet a protein with a specific amino acid chain folds, paradoxically [3] in a matter of seconds, to a particular fold, according to information which must be provided via the underlying linea ...
proteomics - Sigma
... are hydrophilic, surface oriented, and flexible. Most naturally occurring proteins in aqueous solutions have their hydrophilic residues on the surface and their hydrophobic residues buried in the interior. Antibodies bind to epitopes on the surface of proteins. The N- and C-termini of proteins are o ...
... are hydrophilic, surface oriented, and flexible. Most naturally occurring proteins in aqueous solutions have their hydrophilic residues on the surface and their hydrophobic residues buried in the interior. Antibodies bind to epitopes on the surface of proteins. The N- and C-termini of proteins are o ...
Aromatic Amino Acids
... Aromatic amino acids are relatively nonpolar. To different degrees, all aromatic amino acids absorb ultraviolet light. Tyrosine and tryptophan absorb more than do phenylalanine; tryptophan is responsible for most of the absorbance of ultraviolet light (ca. 280 nm) by proteins. Tyrosine is the only o ...
... Aromatic amino acids are relatively nonpolar. To different degrees, all aromatic amino acids absorb ultraviolet light. Tyrosine and tryptophan absorb more than do phenylalanine; tryptophan is responsible for most of the absorbance of ultraviolet light (ca. 280 nm) by proteins. Tyrosine is the only o ...
Protein purification protocol by Dr. Samina Hyder Haq
... (aspartate and glutamate) and the neutral hydrophilic amino acids (asparagine, glutamine, serine, threonine, tyrosine and cysteine). Any compound that interferes with these interactions between amino acid side-chains and water, by reducing the available water, will reduce the solubility of the pro ...
... (aspartate and glutamate) and the neutral hydrophilic amino acids (asparagine, glutamine, serine, threonine, tyrosine and cysteine). Any compound that interferes with these interactions between amino acid side-chains and water, by reducing the available water, will reduce the solubility of the pro ...
Datasheet PDF - BioAssay Systems
... Bradford Colorimetric Protein Determination at 595 nm DESCRIPTION The protein is known as the "building blocks of life" and is one of the most important macromolecules in life science. Proteins are polypeptides made up of amino acids and play various key roles in all aspects of biology. Protein quan ...
... Bradford Colorimetric Protein Determination at 595 nm DESCRIPTION The protein is known as the "building blocks of life" and is one of the most important macromolecules in life science. Proteins are polypeptides made up of amino acids and play various key roles in all aspects of biology. Protein quan ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.