PowerPoint 프레젠테이션
... 4.1 Preliminaries: Wave Motion and Light 4.2 Evidence for Energy Quantization in Atoms 4.3 The Bohr Model: Predicting Discrete Energy Levels in Atoms 4.4 Evidence for Wave-Particle Duality 4.5 The Schrödinger Equation 4.6 Quantum mechanics of Particle-in-a-Box Models General Chemistry I ...
... 4.1 Preliminaries: Wave Motion and Light 4.2 Evidence for Energy Quantization in Atoms 4.3 The Bohr Model: Predicting Discrete Energy Levels in Atoms 4.4 Evidence for Wave-Particle Duality 4.5 The Schrödinger Equation 4.6 Quantum mechanics of Particle-in-a-Box Models General Chemistry I ...
Niels Bohr`s Philosophy of Quantum
... to the production of quantum physical phenomena. But still, these classical forms of causality, as well as their corresponding experimental conditions, can be seen as complementary to one another. And so, "Complementarity" becomes a generalization of "ordinary causality". As I said, the framework of ...
... to the production of quantum physical phenomena. But still, these classical forms of causality, as well as their corresponding experimental conditions, can be seen as complementary to one another. And so, "Complementarity" becomes a generalization of "ordinary causality". As I said, the framework of ...
Optical Spectra and Atomic Structure
... assumed to be capable of sustaining electromagnetic vibrations in much the same manner as an organ pipe is capable of generating a fundamental and its overtones, under appropriate excitation, or as a room is capable of sustaining standing waves. Each harmonic was assumed to be a mode of vibration an ...
... assumed to be capable of sustaining electromagnetic vibrations in much the same manner as an organ pipe is capable of generating a fundamental and its overtones, under appropriate excitation, or as a room is capable of sustaining standing waves. Each harmonic was assumed to be a mode of vibration an ...
Atomic Physics - CAFE SYSTEM CANARIAS
... useful as an intuitive way of thinking about atomic structure and transitions between the energy levels. The ‘proper’ description in terms of atomic wavefunctions is presented in subsequent chapters. Before describing the theory of an atom with one electron, some experimental facts are presented. Th ...
... useful as an intuitive way of thinking about atomic structure and transitions between the energy levels. The ‘proper’ description in terms of atomic wavefunctions is presented in subsequent chapters. Before describing the theory of an atom with one electron, some experimental facts are presented. Th ...
Document
... Note that m does not appear. This makes sense because it just contains information on the direction of the angular momentum. The total angular momentum is relevant so ℓ shows up. To solve this equation we need to know the potential V(r). ...
... Note that m does not appear. This makes sense because it just contains information on the direction of the angular momentum. The total angular momentum is relevant so ℓ shows up. To solve this equation we need to know the potential V(r). ...
Answers to questions on test #2
... Without Slater’s rules: e− #1 spends half its time closer to the nucleus than e− #2. This suggests that e− #1 fells Z = 2 half the time, Z = 1 half the time, and Zef f = 1.5 on average: Zef f ≈ 1.5. (note: this would be true only if, at all times, one e− is much closer to the nucleus than the other. ...
... Without Slater’s rules: e− #1 spends half its time closer to the nucleus than e− #2. This suggests that e− #1 fells Z = 2 half the time, Z = 1 half the time, and Zef f = 1.5 on average: Zef f ≈ 1.5. (note: this would be true only if, at all times, one e− is much closer to the nucleus than the other. ...
Document
... There is a particle in nature called a muon, which has the same charge as the electron but is 207 times heavier. A muon can form a hydrogen-like atom by binding to a proton. ...
... There is a particle in nature called a muon, which has the same charge as the electron but is 207 times heavier. A muon can form a hydrogen-like atom by binding to a proton. ...
The Quantum-Mechanical Model of the Atom
... Classical physics, when applied to black body radiation, predicted that the intensity of the radiation emitted would dramatically increase at shorter and shorter wavelengths. The result was that any hot body should emit intense UV radiation, and even x-rays. Even a human body at 37oC would glow in t ...
... Classical physics, when applied to black body radiation, predicted that the intensity of the radiation emitted would dramatically increase at shorter and shorter wavelengths. The result was that any hot body should emit intense UV radiation, and even x-rays. Even a human body at 37oC would glow in t ...
ppt
... There is a particle in nature called a muon, which has the same charge as the electron but is 207 times heavier. A muon can form a hydrogen-like atom by binding to a proton. ...
... There is a particle in nature called a muon, which has the same charge as the electron but is 207 times heavier. A muon can form a hydrogen-like atom by binding to a proton. ...
Student Text, pp. 650-653
... as a wave phenomenon, was apparently composed of photons possessing distinct particle characteristics. Electrons, to this point thought of as tiny particles with a definite charge and mass, behaved like waves with a definite wavelength, as they moved in orbits within the atom and interacted with obj ...
... as a wave phenomenon, was apparently composed of photons possessing distinct particle characteristics. Electrons, to this point thought of as tiny particles with a definite charge and mass, behaved like waves with a definite wavelength, as they moved in orbits within the atom and interacted with obj ...
Monday, March 3, 2014
... • The electron and hydrogen nucleus actually revolve about their mutual center of mass reduced mass correction!! ...
... • The electron and hydrogen nucleus actually revolve about their mutual center of mass reduced mass correction!! ...
Document
... number. The actual spin angular momentum is S s(s 1) Electrons are s = ½ (spin one-half) particles. Since this never changes, it is often not specified. ms = z-component of spin angular momentum and can have values of ms = −s, −s+1, … s−1, s. The actual z-component of spin angular momentum is S ...
... number. The actual spin angular momentum is S s(s 1) Electrons are s = ½ (spin one-half) particles. Since this never changes, it is often not specified. ms = z-component of spin angular momentum and can have values of ms = −s, −s+1, … s−1, s. The actual z-component of spin angular momentum is S ...
energy levels.
... Based off of the given information, n=4 and ℓ=3. Thus, there are 3 angular nodes present. The total number of nodes in this orbital is: 4-1=3, which means there are no radial nodes present. 1 angular node means ℓ=1 which tells us that we have a p subshell, specifically the pz orbital because the ang ...
... Based off of the given information, n=4 and ℓ=3. Thus, there are 3 angular nodes present. The total number of nodes in this orbital is: 4-1=3, which means there are no radial nodes present. 1 angular node means ℓ=1 which tells us that we have a p subshell, specifically the pz orbital because the ang ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... Electron Arrangement: The Bohr Model When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns t ...
... Electron Arrangement: The Bohr Model When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns t ...
End Show
... model determine about the electrons in an atom? The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations Slide around the nucleus. 6 of 26 © Copyright Pearson Prentice Hall ...
... model determine about the electrons in an atom? The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations Slide around the nucleus. 6 of 26 © Copyright Pearson Prentice Hall ...
Chapter 3. The Structure of the Atom
... recognize the importance of Planck’s quantum hypothesis, but he also pushed it further and with more far-reaching consequences than anybody else. He was a bold thinker who could see through the challenges classical physics faced and came up with imaginative solutions based on the nascent quantum the ...
... recognize the importance of Planck’s quantum hypothesis, but he also pushed it further and with more far-reaching consequences than anybody else. He was a bold thinker who could see through the challenges classical physics faced and came up with imaginative solutions based on the nascent quantum the ...
28 Quantum Physics
... If a wave (EM radiaGon) can behave like a parGcle, might a parGcle act like a wave? Electron can be both an EM wave and a par4cle. Louis de Broglie doctoral thesis 1924 (only 30 pages) – ...
... If a wave (EM radiaGon) can behave like a parGcle, might a parGcle act like a wave? Electron can be both an EM wave and a par4cle. Louis de Broglie doctoral thesis 1924 (only 30 pages) – ...
ATOMIC STRUCTURE
... The three major kinds of particles in an atom are electrons, protons, and neutrons. . The electron is the fundamental unit of negative charge in an atom. The mass of an electron, which equals 1/1836 the mass of a proton, is negligibly small. The proton is the fundamental unit of positive charge in a ...
... The three major kinds of particles in an atom are electrons, protons, and neutrons. . The electron is the fundamental unit of negative charge in an atom. The mass of an electron, which equals 1/1836 the mass of a proton, is negligibly small. The proton is the fundamental unit of positive charge in a ...
YGG-I - UCLA Physics & Astronomy
... Recently evolving ideas in quantum information science have provided a road-map to exploit exotic quantum states to significantly enhance sensor performance. – Sensor noise scales as 1/N where N is the number of particles – “Heisenberg” limit – Shot-noise ~ 1/N1/2 limits existing sensors Challenges: ...
... Recently evolving ideas in quantum information science have provided a road-map to exploit exotic quantum states to significantly enhance sensor performance. – Sensor noise scales as 1/N where N is the number of particles – “Heisenberg” limit – Shot-noise ~ 1/N1/2 limits existing sensors Challenges: ...
ppt
... Electrons occupy orbitals like those of a H atom. Energies of orbitals of many electron atoms are not the same as those for the H atom. Nuclear attraction for electrons is greater as Z increases lowering the electrons’ energy; also have to account for electron-electron repulsion. ...
... Electrons occupy orbitals like those of a H atom. Energies of orbitals of many electron atoms are not the same as those for the H atom. Nuclear attraction for electrons is greater as Z increases lowering the electrons’ energy; also have to account for electron-electron repulsion. ...
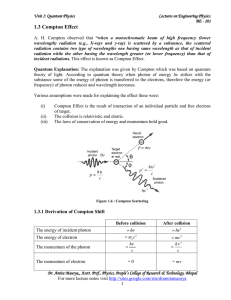
1.3 Compton Effect - IndiaStudyChannel.com
... wavelength) radiation (e.g., X-rays and γ-ray) is scattered by a substance, the scattered radiation contains two type of wavelengths one having same wavelength as that of incident radiation while the other having the wavelength greater (or lower frequency) than that of incident radiations. This effe ...
... wavelength) radiation (e.g., X-rays and γ-ray) is scattered by a substance, the scattered radiation contains two type of wavelengths one having same wavelength as that of incident radiation while the other having the wavelength greater (or lower frequency) than that of incident radiations. This effe ...
Monday, Mar. 23, 2015
... • The electron and hydrogen nucleus actually revolve about their mutual center of mass reduced mass correction!! ...
... • The electron and hydrogen nucleus actually revolve about their mutual center of mass reduced mass correction!! ...
phys3313-fall12
... • “Stationary” states or orbits must exist in atoms, i.e., orbiting electrons do ...
... • “Stationary” states or orbits must exist in atoms, i.e., orbiting electrons do ...
James Franck

James Franck (26 August 1882 – 21 May 1964) was a German physicist who won the 1925 Nobel Prize for Physics with Gustav Hertz ""for their discovery of the laws governing the impact of an electron upon an atom"". He completed his doctorate in 1906 and his habilitation in 1911 at the Frederick William University in Berlin, where he lectured and taught until 1918, having reached the position of professor extraordinarius. He served as a volunteer in the German Army during World War I. He was seriously injured in 1917 in a gas attack and was awarded the Iron Cross 1st Class.Franck became the Head of the Physics Division of the Kaiser Wilhelm Gesellschaft for Physical Chemistry. In 1920, Franck became professor ordinarius of experimental physics and Director of the Second Institute for Experimental Physics at the University of Göttingen. While there he worked on quantum physics with Max Born, who was Director of the Institute of Theoretical Physics. His work included the Franck–Hertz experiment, an important confirmation of the Bohr model of the atom. He promoted the careers of women in physics, notably Lise Meitner, Hertha Sponer and Hilde Levi.After the NSDAP came to power in Germany in 1933, Franck resigned his post in protest against the dismissal of fellow academics. He assisted Frederick Lindemann in helping dismissed Jewish scientists find work overseas, before he left Germany in November 1933. After a year at the Niels Bohr Institute in Denmark, he moved to the United States, where he worked at Johns Hopkins University in Baltimore and then the University of Chicago. During this period he became interested in photosynthesis.Franck participated in the Manhattan Project during World War II as Director of the Chemistry Division of the Metallurgical Laboratory. He was also the chairman of the Committee on Political and Social Problems regarding the atomic bomb, which is best known for the compilation of the Franck Report, which recommended that the atomic bombs not be used on the Japanese cities without warning.