Fundamentals Fall Final Review
... Jade measured out 1 quart of water and added 2 tablespoons of salt. She then brought the water to a boil and measured its maximum temperature. Jade ran two more trials using 2 tablespoons of salt. She then ran 3 trials each with 4 tablespoons of salt and 6 tablespoon of salt. For each trial, Jade us ...
... Jade measured out 1 quart of water and added 2 tablespoons of salt. She then brought the water to a boil and measured its maximum temperature. Jade ran two more trials using 2 tablespoons of salt. She then ran 3 trials each with 4 tablespoons of salt and 6 tablespoon of salt. For each trial, Jade us ...
Chemistry Honors Unit 2 Study Guide Atomic Theory Mr. Brown Use
... mass in grams. So the mole plays an important role in chemistry because it relates mass to the number of atoms or particles. 6.02 X 1023 is called Avogadro ’s number (NA) after the scientist that did this work. ____ 11. Memory Lane: ____ a) Identify and describe the three states of matter solid, liq ...
... mass in grams. So the mole plays an important role in chemistry because it relates mass to the number of atoms or particles. 6.02 X 1023 is called Avogadro ’s number (NA) after the scientist that did this work. ____ 11. Memory Lane: ____ a) Identify and describe the three states of matter solid, liq ...
End of Section A
... 34. In an LCR series circuit the frequency of the a.c. supply is adjusted so that the current registered is a maximum. The values of capacitance and inductance and frequency remain unchanged but the resistance is increased by a factor of four. The current will A. stay the same. B. increase by a fa ...
... 34. In an LCR series circuit the frequency of the a.c. supply is adjusted so that the current registered is a maximum. The values of capacitance and inductance and frequency remain unchanged but the resistance is increased by a factor of four. The current will A. stay the same. B. increase by a fa ...
Resistivity and Drude model
... Resistivities of some common metals, semiconductors, and insulators are given in tables 24.2–4 on textbook pages 866 and 871. There are also plenty of online resources, for example http://www.engineeringtoolbox.com/resistivity-conductivity-d˙418.html. Most metals have resistivities between 1.6·10−8 ...
... Resistivities of some common metals, semiconductors, and insulators are given in tables 24.2–4 on textbook pages 866 and 871. There are also plenty of online resources, for example http://www.engineeringtoolbox.com/resistivity-conductivity-d˙418.html. Most metals have resistivities between 1.6·10−8 ...
Computational Models of Superconducting Quantum Effects
... interpretation of the prohibit energy trenches forming inside of the range of critical temperature and the tendency of the magnetic field to a critical magnetic field in the case of the superconductors of type II. But due the asymmetric inconsistencies in the conceptual map of the superconducting th ...
... interpretation of the prohibit energy trenches forming inside of the range of critical temperature and the tendency of the magnetic field to a critical magnetic field in the case of the superconductors of type II. But due the asymmetric inconsistencies in the conceptual map of the superconducting th ...
Issue Date: November 02, 1998 Newton vs. Einstein: Choosing Your
... Einstein's model focuses on the idea that at the basis of life are subatomic particles that are continually in motion and continually emitting or taking in energy. This model directly challenges the Newtonian model. It states that there is a lot more activity occurring energetically than once believ ...
... Einstein's model focuses on the idea that at the basis of life are subatomic particles that are continually in motion and continually emitting or taking in energy. This model directly challenges the Newtonian model. It states that there is a lot more activity occurring energetically than once believ ...
Chemical Compounds
... How Do I determine the composition and structure of a molecule or a compound ...
... How Do I determine the composition and structure of a molecule or a compound ...
231PHYS
... - Electric Potential: Potential difference and electric potential, potential difference in a uniform electric field, electric potential and potential energy due to point charges, obtaining the value of electric field from the electric potential, electric potential due to continuous charge distributi ...
... - Electric Potential: Potential difference and electric potential, potential difference in a uniform electric field, electric potential and potential energy due to point charges, obtaining the value of electric field from the electric potential, electric potential due to continuous charge distributi ...
Atomic Theories- Part I - Tenafly Public Schools
... Dalton’s Atomic Theory 1. All matter is made of atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. ...
... Dalton’s Atomic Theory 1. All matter is made of atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. ...
Solid State Physics
... As the temperature is lowered below some critical temperature (the Curie temperature), a strong magnetic interaction between the atoms will cause the spins to align within magnetic domains. If the material is cooled within a magnetic field, the domains may be aligned. This spontaneous alignment is d ...
... As the temperature is lowered below some critical temperature (the Curie temperature), a strong magnetic interaction between the atoms will cause the spins to align within magnetic domains. If the material is cooled within a magnetic field, the domains may be aligned. This spontaneous alignment is d ...
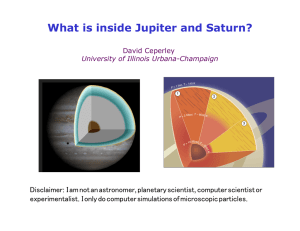
What is inside Jupiter and Saturn? - Physics Illinois
... the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble.” Dirac, 1929 N ei e j ...
... the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble.” Dirac, 1929 N ei e j ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".