Semiconductor Material & Devices
... Electrons in inner shell possess little energy and need a large amount of energy to release Electrons can lose energy in the form of heat and light Free electrons can alco lose and fall into valence shell ...
... Electrons in inner shell possess little energy and need a large amount of energy to release Electrons can lose energy in the form of heat and light Free electrons can alco lose and fall into valence shell ...
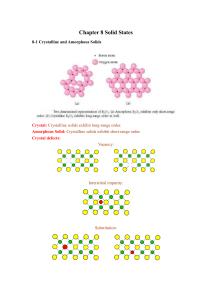
Crystalline solids
... Metallic crystals: Example: iron, copper etc Each atom loses its valence electrons to a common sea of electrons. The crystal is made of positive ion centres. Valence electrons are free to move about the positive ions in the crystal ...
... Metallic crystals: Example: iron, copper etc Each atom loses its valence electrons to a common sea of electrons. The crystal is made of positive ion centres. Valence electrons are free to move about the positive ions in the crystal ...
HW4P1 - Ewp.rpi.edu
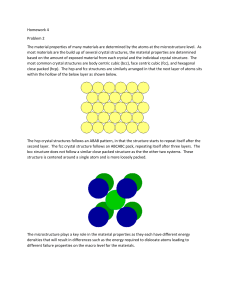
... most materials are the build up of several crystal structures, the material properties are determined based on the amount of exposed material from each crystal and the individual crystal structure. The most common crystal structures are body centric cubic (bcc), face centric cubic (fcc), and hexagon ...
... most materials are the build up of several crystal structures, the material properties are determined based on the amount of exposed material from each crystal and the individual crystal structure. The most common crystal structures are body centric cubic (bcc), face centric cubic (fcc), and hexagon ...
2.3. The response of a PV panel to illumination.
... Elements consist of atoms, with a central nucleus containing uncharged neutrons and positively charged protons. Negatively charged electrons lie outside, and in an electrically neutral atom are equal in number to its protons. Here is a relative scale model. ...
... Elements consist of atoms, with a central nucleus containing uncharged neutrons and positively charged protons. Negatively charged electrons lie outside, and in an electrically neutral atom are equal in number to its protons. Here is a relative scale model. ...
Higher Physics Content Statements
... Higher Physics: Electricity The left hand column below details the content in which students should develop knowledge and understanding. The middle column contains notes, which give further details of the content. The right-hand column gives suggested contexts in which knowledge and understanding an ...
... Higher Physics: Electricity The left hand column below details the content in which students should develop knowledge and understanding. The middle column contains notes, which give further details of the content. The right-hand column gives suggested contexts in which knowledge and understanding an ...
New quasiatomic nanoheterostructures: Superatoms and Excitonic
... critical radius QD а ≥ ас(1) (about 4 nm), will consist entirely with discrete quantum levels. This is called hydrogen-superatom [1]. Localized above the surface of the electron is a valence QD. Quantumdiscrete energy levels superatom thus, are located in the band gap matrices (dielectric or semicon ...
... critical radius QD а ≥ ас(1) (about 4 nm), will consist entirely with discrete quantum levels. This is called hydrogen-superatom [1]. Localized above the surface of the electron is a valence QD. Quantumdiscrete energy levels superatom thus, are located in the band gap matrices (dielectric or semicon ...
classification of materials based on energy band theory
... called semiconductor. The silicon and germanium are the examples of a semiconductor. This does not conduct current at low temperatures but as temperature increases these materials behave as good conductors.. ENERGY BAND DIAGRAMS ...
... called semiconductor. The silicon and germanium are the examples of a semiconductor. This does not conduct current at low temperatures but as temperature increases these materials behave as good conductors.. ENERGY BAND DIAGRAMS ...
Exam 2 Study Concepts
... • Be able to solve all the homework problems without your notes. • Re-do the derivations we did in class on your own. • Equations given: Download formula sheet ...
... • Be able to solve all the homework problems without your notes. • Re-do the derivations we did in class on your own. • Equations given: Download formula sheet ...
Lecture2 - Texas A&M University
... Potential Every particle of mass m raised to a height h above the earth’s surface has a potential energy m.g.h This potential energy can be raised by raising the particle a little higher When the particle is set free, it travels to the point of least potential. ...
... Potential Every particle of mass m raised to a height h above the earth’s surface has a potential energy m.g.h This potential energy can be raised by raising the particle a little higher When the particle is set free, it travels to the point of least potential. ...
Chapter 8 Solid States 8-1 Crystalline and Amorphous Solids
... heavily doped. And the depletion region is very narrow. Fig. (a) No bias: EF(p)=EF(n), electrons tunnel in both direction are equal. Fig. (b) Small forward bias (From point a→b→c of I-V curve): Electrons tunnel from n-region to p-region only, because the filled lower part of n-region conduction band ...
... heavily doped. And the depletion region is very narrow. Fig. (a) No bias: EF(p)=EF(n), electrons tunnel in both direction are equal. Fig. (b) Small forward bias (From point a→b→c of I-V curve): Electrons tunnel from n-region to p-region only, because the filled lower part of n-region conduction band ...
conduction band
... A discriminator rejects all pulses below a certain level which is set by applying a discriminator bias voltage ...
... A discriminator rejects all pulses below a certain level which is set by applying a discriminator bias voltage ...
Energy Bands: • The Fermi level: o This is an energy level in the
... o In the absence of current flaw, the Fermi-level would be continuous and constant. • Energy Bands under Bias: o An applied electric field pushes the bands in its direction, e.g ...
... o In the absence of current flaw, the Fermi-level would be continuous and constant. • Energy Bands under Bias: o An applied electric field pushes the bands in its direction, e.g ...