CHAPTER 11: Semiconductor Theory and Devices
... energy. A good way to think of the solar cell is to consider the LED in reverse (Figure 11.18). A pnjunction diode can absorb a photon of solar radiation by having an electron make a transition from the valence band to the conduction band. In doing so, both a conducting electron and a hole have been ...
... energy. A good way to think of the solar cell is to consider the LED in reverse (Figure 11.18). A pnjunction diode can absorb a photon of solar radiation by having an electron make a transition from the valence band to the conduction band. In doing so, both a conducting electron and a hole have been ...
Periodic Trends Lab CHM120 1The Periodic Table is one of the
... The melting point of most metals is quite high so we know there must be substantial forces holding them together. The classic description of these structures involves a lattice of metal atoms in a sea of delocalized electrons, and one can visualize that coulombic forces of attraction between the ele ...
... The melting point of most metals is quite high so we know there must be substantial forces holding them together. The classic description of these structures involves a lattice of metal atoms in a sea of delocalized electrons, and one can visualize that coulombic forces of attraction between the ele ...
ALUMINUM AND ITS ALLOYS - redemat
... impurities. As is the case with semiconductors, these polymers may be made either n-type (i.e., free-electron charge carriers) or p-type (i.e., electron-hole charge carriers) depending on the dopant. However, unlike semiconductors, the dopant atoms or molecules do not substitute for or replace any o ...
... impurities. As is the case with semiconductors, these polymers may be made either n-type (i.e., free-electron charge carriers) or p-type (i.e., electron-hole charge carriers) depending on the dopant. However, unlike semiconductors, the dopant atoms or molecules do not substitute for or replace any o ...
CHAPTER 11: Semiconductor Theory and Devices
... For an infinite lattice the allowed energies within each band are continuous rather than discrete. In a real crystal the lattice is not infinite, but even if chains are thousands of atoms long, the allowed energies are nearly continuous. ...
... For an infinite lattice the allowed energies within each band are continuous rather than discrete. In a real crystal the lattice is not infinite, but even if chains are thousands of atoms long, the allowed energies are nearly continuous. ...
Introduction and Semiconductor Technology
... To make semiconductors better conductors, add impurities (dopants) to contribute extra electrons or extra holes – elements with 5 outer electrons contribute an extra electron to the lattice (donor dopant) – elements with 3 outer electrons accept an electron from the silicon (acceptor dopant) ...
... To make semiconductors better conductors, add impurities (dopants) to contribute extra electrons or extra holes – elements with 5 outer electrons contribute an extra electron to the lattice (donor dopant) – elements with 3 outer electrons accept an electron from the silicon (acceptor dopant) ...
Bonding in Metals and Semiconductors
... required to induce a minimum current to flow in these semiconducting materials. These observations will provide us with a feel for how the composition of these diodes influences their Band-Gap energies. Next, we will observe the change induced when these diodes are cooled to liquid Nitrogen temperat ...
... required to induce a minimum current to flow in these semiconducting materials. These observations will provide us with a feel for how the composition of these diodes influences their Band-Gap energies. Next, we will observe the change induced when these diodes are cooled to liquid Nitrogen temperat ...
Electricity - people.vcu.edu
... • Protons are positively charged – relatively, positively charged things are stationary ...
... • Protons are positively charged – relatively, positively charged things are stationary ...
MOS Transistor Theory
... The electron affinity of Silicon which is the potential difference between the conduction band and the vacuum (free space) is expressed as qχ. The energy required to move an electron from the Fermi Level into free space is called the work function qΦS and is given by: qΦS= qχ+EC-EF If we bring the g ...
... The electron affinity of Silicon which is the potential difference between the conduction band and the vacuum (free space) is expressed as qχ. The energy required to move an electron from the Fermi Level into free space is called the work function qΦS and is given by: qΦS= qχ+EC-EF If we bring the g ...
Conduction electrons
... • Intermediate resistivity => “semiconductor” – conductivity lies between that of conductors and insulators – generally crystalline in structure for IC devices • In recent years, however, non-crystalline semiconductors have become commercially very important ...
... • Intermediate resistivity => “semiconductor” – conductivity lies between that of conductors and insulators – generally crystalline in structure for IC devices • In recent years, however, non-crystalline semiconductors have become commercially very important ...
Conductors, semiconductors and insulators
... An electron from the next atom can move into the hole created, as described previously. Conduction can thus take place by the movement of positive holes. This is called a p-type semiconductor, as most conduction takes place by the movement of positively charged holes. Notes on doping The doping ma ...
... An electron from the next atom can move into the hole created, as described previously. Conduction can thus take place by the movement of positive holes. This is called a p-type semiconductor, as most conduction takes place by the movement of positively charged holes. Notes on doping The doping ma ...
Understanding Solid State Physics Additional Questions
... 6.6(a) Does the resistivity of a metal increase or decrease with temperature? 6.6(b) Why is this? 6.6(c) What other non thermal factors contribute to the resistivity? 6.7(a) For metals and alloys, what is unusual about dividing the thermal conductivity of each solid by its electrical conductivity? 6 ...
... 6.6(a) Does the resistivity of a metal increase or decrease with temperature? 6.6(b) Why is this? 6.6(c) What other non thermal factors contribute to the resistivity? 6.7(a) For metals and alloys, what is unusual about dividing the thermal conductivity of each solid by its electrical conductivity? 6 ...
Periodic Table Test – Study Guide What state of matter are almost all
... Who published the first periodic table? Mendeleev How did Dobereiner classify elements? similar properties How did he place these elements together? in triads (groups of 3) with increasing atomic mass Why did Mendeleev leave blank spaces on the periodic table? for undiscovered elements What does the ...
... Who published the first periodic table? Mendeleev How did Dobereiner classify elements? similar properties How did he place these elements together? in triads (groups of 3) with increasing atomic mass Why did Mendeleev leave blank spaces on the periodic table? for undiscovered elements What does the ...
Class28_review
... Our Model of the Atom • If the atom is in the “ground state” of lowest energy, electrons fill the states in the lowest available energy levels. The first shell has two possible states, and the second shell has eight possible states. Higher shells have more states, but we’ll represent them with the ...
... Our Model of the Atom • If the atom is in the “ground state” of lowest energy, electrons fill the states in the lowest available energy levels. The first shell has two possible states, and the second shell has eight possible states. Higher shells have more states, but we’ll represent them with the ...
word document
... somewhere in the energy gap between the two. For p type semiconductors, the Fermi level is close to but above the valence band; for n type semiconductors the Fermi level is close to but below the conduction band. For intrinsic semiconductors, the Fermi level is close to the middle between the bands. ...
... somewhere in the energy gap between the two. For p type semiconductors, the Fermi level is close to but above the valence band; for n type semiconductors the Fermi level is close to but below the conduction band. For intrinsic semiconductors, the Fermi level is close to the middle between the bands. ...
IV DETECTORS
... • electron energy levels of atoms in a solid split and merge into energy bands with many sub-levels • atoms are kept together by ‘valence electrons’ in the outer shells; combined energy levels of those shells form the valence band • excitation can move valence band electrons to higher energy levels ...
... • electron energy levels of atoms in a solid split and merge into energy bands with many sub-levels • atoms are kept together by ‘valence electrons’ in the outer shells; combined energy levels of those shells form the valence band • excitation can move valence band electrons to higher energy levels ...
III.5. THE THERMISTOR 1. Work purpose To check the law
... To check the law describing the temperature dependence of the electrical resistance of semiconductor materials and to evaluate their gap. 2. Theory Quantum mechanics states that isolated microsystems (electrons, molecules, ions) have only discrete energy spectra among which transitions are possible. ...
... To check the law describing the temperature dependence of the electrical resistance of semiconductor materials and to evaluate their gap. 2. Theory Quantum mechanics states that isolated microsystems (electrons, molecules, ions) have only discrete energy spectra among which transitions are possible. ...
Solid State Physics - University of Surrey
... Upon contact the excess electrons in the n-doped region diffuse into the p-doped region, and the excess holes in the p-doped region diffuse into the n-doped region and recombine. This reduces the concentration gradient at the interface. The Fermi-levels align (thermal equilibrium) Ionised donor and ...
... Upon contact the excess electrons in the n-doped region diffuse into the p-doped region, and the excess holes in the p-doped region diffuse into the n-doped region and recombine. This reduces the concentration gradient at the interface. The Fermi-levels align (thermal equilibrium) Ionised donor and ...
(915MHz and 2450 MHz) Harvester
... are forming the basis of radio communication can be used as an energy source is a recent idea and one of the most exciting areas of research. Because in the case of an electromagnetic harvester with sufficient efficiency and output power developed, without the need for batteries and by getting rid o ...
... are forming the basis of radio communication can be used as an energy source is a recent idea and one of the most exciting areas of research. Because in the case of an electromagnetic harvester with sufficient efficiency and output power developed, without the need for batteries and by getting rid o ...
A Student Introduction to Solar Energy
... absorbed but will traverse the material without interaction. In a real semiconductor, the valence and conduction bands are not flat, but vary depending on the so-called k-vector that describes the momentum of an electron in the semiconductor. This means, that the energy of an electron is dependent on ...
... absorbed but will traverse the material without interaction. In a real semiconductor, the valence and conduction bands are not flat, but vary depending on the so-called k-vector that describes the momentum of an electron in the semiconductor. This means, that the energy of an electron is dependent on ...
ee201 semiconductor device chp1
... There are 2 types of Extrinsic Material: n-type & p-type. Both materials are formed by adding a predetermined no. of impurity atoms to a Si base. n-type: created by introducing impurity elements that have 5 valence e- (eg. As & P). p-type: formed by doping a pure Ge or Si crystal with impurity atoms ...
... There are 2 types of Extrinsic Material: n-type & p-type. Both materials are formed by adding a predetermined no. of impurity atoms to a Si base. n-type: created by introducing impurity elements that have 5 valence e- (eg. As & P). p-type: formed by doping a pure Ge or Si crystal with impurity atoms ...
Ceramics Ceramics are inorganic and nonmetallic materials
... Because ceramics are composed of at least two elements, and often more, their crystal structures are generally more complex than those for metals Crystal Structures For those ceramic materials for which the atomic bonding is predominantly ionic, the crystal structures may be thought of as being comp ...
... Because ceramics are composed of at least two elements, and often more, their crystal structures are generally more complex than those for metals Crystal Structures For those ceramic materials for which the atomic bonding is predominantly ionic, the crystal structures may be thought of as being comp ...
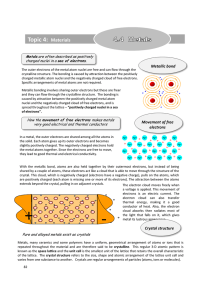
Topic 4: Materials - Education Umbrella
... the metal atoms together. Since the electrons are free to move, they lead to good thermal and electrical conductivity. ...
... the metal atoms together. Since the electrons are free to move, they lead to good thermal and electrical conductivity. ...
phys3313-fall12-112612
... • The band structures of insulators and semiconductors resemble each other qualitatively. Normally there exists in both insulators and semiconductors a filled energy band (referred to as the valence band) separated from the next higher band (referred to as the conduction band) by an energy gap. • If ...
... • The band structures of insulators and semiconductors resemble each other qualitatively. Normally there exists in both insulators and semiconductors a filled energy band (referred to as the valence band) separated from the next higher band (referred to as the conduction band) by an energy gap. • If ...
Technical terms-3
... Electron energy band A series of electron energy states that are very closely spaced with respect to energy. Electroneutrality The state of having exactly the same numbers of positive and negative electrical charges (ionic and electronic), that is, of being electrically neutral. Electron state (lev ...
... Electron energy band A series of electron energy states that are very closely spaced with respect to energy. Electroneutrality The state of having exactly the same numbers of positive and negative electrical charges (ionic and electronic), that is, of being electrically neutral. Electron state (lev ...