Ayres, Bob – Exergy, economic growth and degrowth
... growth, can be met by increased supply at no increase in price. (This was explicit in the 2010 IMF forecast and all IEA and EIA forecasts up to 2006.) It implies that the energy return on energy investments (EROEI) will be constant over time. ...
... growth, can be met by increased supply at no increase in price. (This was explicit in the 2010 IMF forecast and all IEA and EIA forecasts up to 2006.) It implies that the energy return on energy investments (EROEI) will be constant over time. ...
Fluorescence * a key to unravel (atomic) structure and dynamics
... ….but nobody has tried to measure this beautiful spectra yet. ...
... ….but nobody has tried to measure this beautiful spectra yet. ...
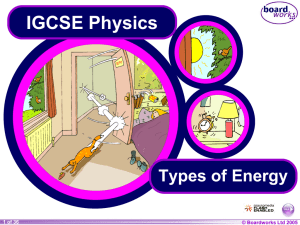
Types of Energy - AIS IGCSE Science
... energy. They can move past each other but are still held together. ...
... energy. They can move past each other but are still held together. ...
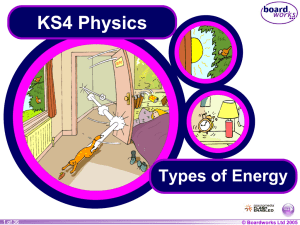
Types of Energy - We can`t sign you in
... energy. They can move past each other but are still held together. ...
... energy. They can move past each other but are still held together. ...
All the 5`s - The Physics Teacher
... The Principle of Conservation of Momentum states that in any collision between two objects, the total momentum before impact equals total momentum after impact, provided no external forces act on the system. (c) Explain why heat does not travel through solids by means of convection. The particles ca ...
... The Principle of Conservation of Momentum states that in any collision between two objects, the total momentum before impact equals total momentum after impact, provided no external forces act on the system. (c) Explain why heat does not travel through solids by means of convection. The particles ca ...
Chemistry/Physical Science - Thermodynamics
... (1) Can predict changes in entropy associated w/ changes in phases (2) Entropy increases w/ increase in KE (3) Dissolving gas in a solvent results in decrease in S (4) Increase in T causes an increase in S (5) If no change in phase, S of system increases when number of gaseous product particles is g ...
... (1) Can predict changes in entropy associated w/ changes in phases (2) Entropy increases w/ increase in KE (3) Dissolving gas in a solvent results in decrease in S (4) Increase in T causes an increase in S (5) If no change in phase, S of system increases when number of gaseous product particles is g ...
Lecture 2
... their roughness and reflection coefficient. So it is not truly perfect, but can clearly be made close enough that we cannot measure the difference. The container, or more specifically the hole is called a BLACK BODY ...
... their roughness and reflection coefficient. So it is not truly perfect, but can clearly be made close enough that we cannot measure the difference. The container, or more specifically the hole is called a BLACK BODY ...
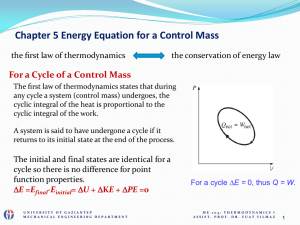
ME 204 Thermodynamics I
... control surfaces for various work modes or use the first law or conservation of mass)? (determination of properties from the relation between them) vii) what we have done so far in previous steps, how do we proceed to find whatever it is that is desired? Is a trial-anderror solution necessary? (anot ...
... control surfaces for various work modes or use the first law or conservation of mass)? (determination of properties from the relation between them) vii) what we have done so far in previous steps, how do we proceed to find whatever it is that is desired? Is a trial-anderror solution necessary? (anot ...
Thermodynamics for Systems Biology
... that is completely “thermalized”, i.e. as randomized as the temperature allows. Thermal energy at a high temperature is more ordered than thermal energy at a low temperature. In this connection, work can be viewed as heat from an infinite temperature system, i.e. not randomized at all but rather di ...
... that is completely “thermalized”, i.e. as randomized as the temperature allows. Thermal energy at a high temperature is more ordered than thermal energy at a low temperature. In this connection, work can be viewed as heat from an infinite temperature system, i.e. not randomized at all but rather di ...
Chapter 5. Thermochemistry.
... thermodynamics, we cannot think about the whole universe at one time. We have to think about the system of interest to us, which for chemistry is usually the contents of something the size of a beaker. Thermodynamics is the bookkeeping of energy, and so we ...
... thermodynamics, we cannot think about the whole universe at one time. We have to think about the system of interest to us, which for chemistry is usually the contents of something the size of a beaker. Thermodynamics is the bookkeeping of energy, and so we ...
thermodynamics - La Salle High School
... Convention: Positive: heat flows into system work done onto system Negative: heat flows out of system work done by system ...
... Convention: Positive: heat flows into system work done onto system Negative: heat flows out of system work done by system ...
Energy Meter Working Principle
... current to be used. It comprises a metal disc which is free to rotate between them and two electromagnets. Current pulled through the building's electrical circuits powers directly by current in the incoming power lines; the other, one electromagnet. The interaction causes the disc to rotate. Two pe ...
... current to be used. It comprises a metal disc which is free to rotate between them and two electromagnets. Current pulled through the building's electrical circuits powers directly by current in the incoming power lines; the other, one electromagnet. The interaction causes the disc to rotate. Two pe ...
Lesson Plan Title: Transformations: The Many Forms of Energy
... problem for the mass in kg and the height in meters. Once that is found they will be able to input the numbers and units with the gravity constant and solve using PE=mgh. Students will also follow the same process to solve for Kinetic energy, using the formula: KE= ½ MassV2, Once again m= mass measu ...
... problem for the mass in kg and the height in meters. Once that is found they will be able to input the numbers and units with the gravity constant and solve using PE=mgh. Students will also follow the same process to solve for Kinetic energy, using the formula: KE= ½ MassV2, Once again m= mass measu ...
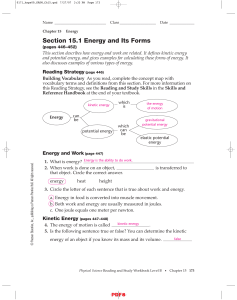
Section 15.1 Energy and Its Forms IPLS
... 7. Circle the letter of the type of energy that increases as the pole bends before it propels a pole-vaulter up into the air. a. kinetic energy b. mechanical energy c. elastic potential energy 8. Is the following sentence true or false? For a mechanical change in an isolated system, the mechanical e ...
... 7. Circle the letter of the type of energy that increases as the pole bends before it propels a pole-vaulter up into the air. a. kinetic energy b. mechanical energy c. elastic potential energy 8. Is the following sentence true or false? For a mechanical change in an isolated system, the mechanical e ...
A Paradox Related to Mechanical and Electrical Energy Conversion
... where the minus sign means the net force acting on the plate is pointing to the left, which is NOT the result provided by Idea 1. So if the movable plate is stationary at the beginning, it will move toward the fixed plate. In this process, the energy of the system is increasing. The movable plate is ...
... where the minus sign means the net force acting on the plate is pointing to the left, which is NOT the result provided by Idea 1. So if the movable plate is stationary at the beginning, it will move toward the fixed plate. In this process, the energy of the system is increasing. The movable plate is ...
Solid State Physics
... At temperatures above 0 K, only the populations of states with energies near the Fermi energy are changed. Collisions with lattice ions can’t deliver enough energy to most of the electrons to bump them up into any of the unoccupied states as long as the temperature is far below the Fermi Temperature ...
... At temperatures above 0 K, only the populations of states with energies near the Fermi energy are changed. Collisions with lattice ions can’t deliver enough energy to most of the electrons to bump them up into any of the unoccupied states as long as the temperature is far below the Fermi Temperature ...
Energy Use - Effingham County Schools
... Thermal energy is the internal energy in substances - the vibration and movement of atoms and molecules within substance. Thermal energy is created in the movement of atoms. ...
... Thermal energy is the internal energy in substances - the vibration and movement of atoms and molecules within substance. Thermal energy is created in the movement of atoms. ...
Conservation of energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can be neither created nor be destroyed, but it transforms from one form to another, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite.A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist. That is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.