Lesson #5 – Electric Potential
... Electrical engineers and electronic technicians often work on devices that are capable of severely shocking or even killing a person. In order to reduce the possibility of this hazard, they often tie the common (negative) lead to a single reference point on the circuit called “ground.” The electric ...
... Electrical engineers and electronic technicians often work on devices that are capable of severely shocking or even killing a person. In order to reduce the possibility of this hazard, they often tie the common (negative) lead to a single reference point on the circuit called “ground.” The electric ...
Basic Physical Quantities and Laws
... All the terms used in science must be defined carefully to avoid possible confusion and misunderstanding. As you will see, once a term has been defined, it is used to define still other terms, and in this manner the scientific jargon is formulated. However, there must be a starting point; that is, s ...
... All the terms used in science must be defined carefully to avoid possible confusion and misunderstanding. As you will see, once a term has been defined, it is used to define still other terms, and in this manner the scientific jargon is formulated. However, there must be a starting point; that is, s ...
Chapter 5 Thermochemistry
... • Reactions can be carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education ...
... • Reactions can be carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education ...
unit ii chemical thermodynamics
... First law of thermodynamics: The law of conservation of energy. Energy can be neither created nor destroyed, but it can be converted from one form to another. The mathematical form of First law of thermodynamics is ΔE = q – w where ΔE, q and w represent respectively the change in internal energy, q ...
... First law of thermodynamics: The law of conservation of energy. Energy can be neither created nor destroyed, but it can be converted from one form to another. The mathematical form of First law of thermodynamics is ΔE = q – w where ΔE, q and w represent respectively the change in internal energy, q ...
physics
... All of your answers are to be recorded on the separate answer paper. For each question in Part I and Part II, decide which of the choices given is the best answer. Then on the answer paper, in the row of numbers for that question, circle with pencil the number of the choice that you have selected. T ...
... All of your answers are to be recorded on the separate answer paper. For each question in Part I and Part II, decide which of the choices given is the best answer. Then on the answer paper, in the row of numbers for that question, circle with pencil the number of the choice that you have selected. T ...
Egely Wheel® Vitality Indicator
... field around a static, stationary electric charge. But when the same electric charge starts moving, there is a magnetic field around it. What happens when the charge rotates? Then a new kind of field appears, which is similar to the electric and magnetic field: termed as rotating or rather “spin” fi ...
... field around a static, stationary electric charge. But when the same electric charge starts moving, there is a magnetic field around it. What happens when the charge rotates? Then a new kind of field appears, which is similar to the electric and magnetic field: termed as rotating or rather “spin” fi ...
fluid flow - AuroEnergy
... In simplest terms, the Laws of Thermodynamics dictate the specifics for the movement of heat and work. Basically, the First Law is a statement of the conservation of energy – the Second Law is a statement about the quality of energy or direction of that conservation – and the Third Law is a statemen ...
... In simplest terms, the Laws of Thermodynamics dictate the specifics for the movement of heat and work. Basically, the First Law is a statement of the conservation of energy – the Second Law is a statement about the quality of energy or direction of that conservation – and the Third Law is a statemen ...
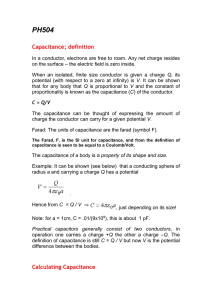
PH504lec0809-6
... When an isolated, finite size conductor is given a charge Q, its potential (with respect to a zero at infinity) is V. It can be shown that for any body that Q is proportional to V and the constant of proportionality is known as the capacitance (C) of the conductor. C = Q/V The capacitance can be tho ...
... When an isolated, finite size conductor is given a charge Q, its potential (with respect to a zero at infinity) is V. It can be shown that for any body that Q is proportional to V and the constant of proportionality is known as the capacitance (C) of the conductor. C = Q/V The capacitance can be tho ...
437913 Forces and Motion Energy Conservation and
... 1. Simple machines are often used to reduce the amount of force. 2. The work output from a simple machine is always less than the work input to the simple machine. 3. Work is a means of transferring energy. 4. Energy is always conserved. Based on this information, which statement is a valid conclusi ...
... 1. Simple machines are often used to reduce the amount of force. 2. The work output from a simple machine is always less than the work input to the simple machine. 3. Work is a means of transferring energy. 4. Energy is always conserved. Based on this information, which statement is a valid conclusi ...
Bohr atom.latex - for blackholeformulas.com
... protons orbiting on their circular paths. The sine waves are an edge view of the orbital plane and currents traced out by the electron and proton pair as they move across the page on a helical path like a spring on a string. Both orbit each other on opposite sides of the center of mass of the system ...
... protons orbiting on their circular paths. The sine waves are an edge view of the orbital plane and currents traced out by the electron and proton pair as they move across the page on a helical path like a spring on a string. Both orbit each other on opposite sides of the center of mass of the system ...
Principles of Energy Conversion
... gasoline are required over the lifetime of an automobile, which corresponds to 109 kJc , or 21 142 kg of gasoline2 (46 610 lbm = 23.5 tons). If the nuclear energy stored as mass were used instead of chemical energy, then only 0.1 µg (2.5 × 10−7 lbm) of gasoline would be required, but the stored nucl ...
... gasoline are required over the lifetime of an automobile, which corresponds to 109 kJc , or 21 142 kg of gasoline2 (46 610 lbm = 23.5 tons). If the nuclear energy stored as mass were used instead of chemical energy, then only 0.1 µg (2.5 × 10−7 lbm) of gasoline would be required, but the stored nucl ...
WRL1834.tmp - Symposium on Chemical Physics
... This postulate has its most profound basis in the microscopic laws of physics. One can use either classical or quantum mechanics. In either case the system as a whole evolves in time and it satisfies the law of conservation of energy. This is true only for conservative systems, but this suffices as ...
... This postulate has its most profound basis in the microscopic laws of physics. One can use either classical or quantum mechanics. In either case the system as a whole evolves in time and it satisfies the law of conservation of energy. This is true only for conservative systems, but this suffices as ...
Fundamentals of Equilibrium Thermodynamics
... This postulate has its most profound basis in the microscopic laws of physics. One can use either classical or quantum mechanics. In either case the system as a whole evolves in time and it satisfies the law of conservation of energy. This is true only for conservative systems, but this suffices as ...
... This postulate has its most profound basis in the microscopic laws of physics. One can use either classical or quantum mechanics. In either case the system as a whole evolves in time and it satisfies the law of conservation of energy. This is true only for conservative systems, but this suffices as ...
POTENTIAL 1. A uniform electric field with a magnitude of 500 N/C is
... 3. When an electron is brought near a negatively charged sphere, its potential energy increases. The reason this happens is that a. negative charges repel each other. b. work was done to bring the charges together. c. two like charges go from a position far apart to a position close together. d. non ...
... 3. When an electron is brought near a negatively charged sphere, its potential energy increases. The reason this happens is that a. negative charges repel each other. b. work was done to bring the charges together. c. two like charges go from a position far apart to a position close together. d. non ...
W05D1_Conductors and Insulators_mac_v03_jwb
... Exam One will be handed back at the end of class on W05D1 Mon and Tuesday. Students can take the exam home and compare with solutions. Regrading Requests: Exams must be returned with a request to regrade in class on W05D2 Wed or Thurs The student must clearly indicate on the cover sheet which proble ...
... Exam One will be handed back at the end of class on W05D1 Mon and Tuesday. Students can take the exam home and compare with solutions. Regrading Requests: Exams must be returned with a request to regrade in class on W05D2 Wed or Thurs The student must clearly indicate on the cover sheet which proble ...
Physics 30 - Paul Rowe JrSr High School
... Unit C: Electromagnetic Radiation Explain the nature and behaviour of EMR, using the wave model. describe, qualitatively, how all accelerating charges produce EMR compare and contrast the constituents of the electromagnetic spectrum on the basis of frequency and ...
... Unit C: Electromagnetic Radiation Explain the nature and behaviour of EMR, using the wave model. describe, qualitatively, how all accelerating charges produce EMR compare and contrast the constituents of the electromagnetic spectrum on the basis of frequency and ...
Conservation of energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can be neither created nor be destroyed, but it transforms from one form to another, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite.A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist. That is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.