chapter 19 - Celina City Schools
... a) ____________ - a machine designed to generate enough energy to study quarks Located in Batavia, IL (The Fermi National Accelerator Laboratory) The machine is also known as a ____________ IV. Models—Tools for Scientists (timeline of contributions leading to the discovery of the atom) Greeks ...
... a) ____________ - a machine designed to generate enough energy to study quarks Located in Batavia, IL (The Fermi National Accelerator Laboratory) The machine is also known as a ____________ IV. Models—Tools for Scientists (timeline of contributions leading to the discovery of the atom) Greeks ...
File
... quantum theory to Rutherford’s atomic structure by assuming that electrons travel in stationary orbits defined by their angular momentum. This led to the calculation of possible energy levels for these orbits and the postulation that the emission of light occurs when an electron moves into a lower e ...
... quantum theory to Rutherford’s atomic structure by assuming that electrons travel in stationary orbits defined by their angular momentum. This led to the calculation of possible energy levels for these orbits and the postulation that the emission of light occurs when an electron moves into a lower e ...
Atomic Structure/Atomic Theory
... Valence electrons are electrons in an atoms outermost electron energy level and determine how the atom reacts with other atoms ...
... Valence electrons are electrons in an atoms outermost electron energy level and determine how the atom reacts with other atoms ...
creator of the atomic theory
... moved in paths at certain distances around the nucleus, called orbitals. Higher orbitals have more energy. When electrons move down orbitals, light (photons) is emitted. ...
... moved in paths at certain distances around the nucleus, called orbitals. Higher orbitals have more energy. When electrons move down orbitals, light (photons) is emitted. ...
Chemistry Honors Semester One Final Exam Review 2014 1. Define
... 58. A prospector finds 39.39 g of pure gold (atomic mass 196.9665 amu). How many atoms are there? 59. Visible light, X rays, infrared radiation, and radio waves all have the same ________. 60. For electromagnetic radiation, what does c (the speed of light) equal? 61. Because c, the speed of electrom ...
... 58. A prospector finds 39.39 g of pure gold (atomic mass 196.9665 amu). How many atoms are there? 59. Visible light, X rays, infrared radiation, and radio waves all have the same ________. 60. For electromagnetic radiation, what does c (the speed of light) equal? 61. Because c, the speed of electrom ...
Atomic Theory, cont*d
... The beams were bouncing off the positively charged core of the atoms. Rutherford originally called this a proton, because it was positively charged. Later, it was renamed the nucleus. The nucleus of the atom has almost all the mass of the atom. Therefore, most of an atom is empty space. ...
... The beams were bouncing off the positively charged core of the atoms. Rutherford originally called this a proton, because it was positively charged. Later, it was renamed the nucleus. The nucleus of the atom has almost all the mass of the atom. Therefore, most of an atom is empty space. ...
File - Rogers` Rocket Science
... different from those of any other element. 3) Atoms of different elements __________in simple ________-number ratios to form _____________ compounds. 4) In chemical reactions, atoms are_________________, ________________, or ____________– but never changed into atoms of another element. Sizing up th ...
... different from those of any other element. 3) Atoms of different elements __________in simple ________-number ratios to form _____________ compounds. 4) In chemical reactions, atoms are_________________, ________________, or ____________– but never changed into atoms of another element. Sizing up th ...
Chapter 2. Atoms, Molecules and Ions
... Discovery of the Nucleus: Plum Pudding atomic model. Ernest Rutherford’s experiment showing small size, hardness and positive charge of the nucleus. Modern view of the atom with most mass concentrated in the nucleus and the size due to orbiting electrons. Discovery of the Proton: Magnitude of + char ...
... Discovery of the Nucleus: Plum Pudding atomic model. Ernest Rutherford’s experiment showing small size, hardness and positive charge of the nucleus. Modern view of the atom with most mass concentrated in the nucleus and the size due to orbiting electrons. Discovery of the Proton: Magnitude of + char ...
Nature of Matter
... • If we change the atomic number, we change the element we are talking about… ...
... • If we change the atomic number, we change the element we are talking about… ...
A quick summary about atoms: Atomic masses and/or hydrogen
... In 1905 Albert Einstein proved that atoms must exist (he showed that Brownian Motion would look different if there weren't really atoms and ...
... In 1905 Albert Einstein proved that atoms must exist (he showed that Brownian Motion would look different if there weren't really atoms and ...
Atomic terms - ATOMIC NUMBER: The number of protons in the
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
In 1908 Ernest Rutherford fired a stream of positively
... foil and found that most of them passed right through while others hit something and bounced away. What would cause something to repel a positive charge? Another positive charge. ...
... foil and found that most of them passed right through while others hit something and bounced away. What would cause something to repel a positive charge? Another positive charge. ...
Chemistry Review
... Energy Levels: region around the nucleus where the electron is likely to be moving. a ladder that isn’t equally spaced further the distance, closer the spacing the higher the energy level the farther it is from the nucleus Electrons can jump from 1 energy level to another. ...
... Energy Levels: region around the nucleus where the electron is likely to be moving. a ladder that isn’t equally spaced further the distance, closer the spacing the higher the energy level the farther it is from the nucleus Electrons can jump from 1 energy level to another. ...
Chapter 8: Chemical Bonding
... Atoms tend to gain, lose or share e- to get to the nearest noble gas configuration Noble gases: all (except He) have s2p6 valence shells (8 e-) ...
... Atoms tend to gain, lose or share e- to get to the nearest noble gas configuration Noble gases: all (except He) have s2p6 valence shells (8 e-) ...
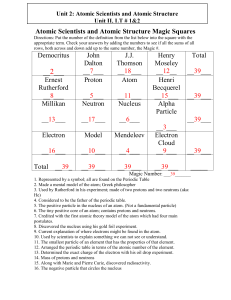
Atomic Scientists and Atomic Structure Magic Squares
... 6. The tiny positive core of an atom; contains protons and neutrons. 7. Credited with the first atomic theory model of the atom which had four main postulates. 8. Discovered the nucleus using his gold foil experiment. 9. Current explanation of where electrons might be found in the atom. 10. Used by ...
... 6. The tiny positive core of an atom; contains protons and neutrons. 7. Credited with the first atomic theory model of the atom which had four main postulates. 8. Discovered the nucleus using his gold foil experiment. 9. Current explanation of where electrons might be found in the atom. 10. Used by ...
Chemistry Common Assessment Quarter One
... ____ 40. To what category of elements does an element belong if it is a poor conductor of electricity? a. transition elements c. nonmetals b. metalloids d. metals ____ 41. Which of the following is true about the electron configurations of the noble gases? a. The highest occupied s and p sublevels ...
... ____ 40. To what category of elements does an element belong if it is a poor conductor of electricity? a. transition elements c. nonmetals b. metalloids d. metals ____ 41. Which of the following is true about the electron configurations of the noble gases? a. The highest occupied s and p sublevels ...
Chemistry Common Assessment Quarter One
... b. Atoms of different elements always combine in one-to-one ratios. c. Atoms of the same element are always identical. d. Individual atoms can be seen with a microscope. 3. Why did J. J. Thomson reason that electrons must be a part of the atoms of all elements? a. Cathode rays are negatively-charged ...
... b. Atoms of different elements always combine in one-to-one ratios. c. Atoms of the same element are always identical. d. Individual atoms can be seen with a microscope. 3. Why did J. J. Thomson reason that electrons must be a part of the atoms of all elements? a. Cathode rays are negatively-charged ...
Atoms and the Periodic Table PowerPoint
... in the table, he predicted a new element would one day be found and deduced its properties. And he was right. Three of those elements were found during his lifetime: gallium, scandium, and germanium. ...
... in the table, he predicted a new element would one day be found and deduced its properties. And he was right. Three of those elements were found during his lifetime: gallium, scandium, and germanium. ...
Chapter 1 Notes
... • Early scientists theorized that eventually you would not be able to cut it in half any more. o Only one particle would be left. o They named these particles ‘Atoms’ • Atoms means ‘cannot be divided’ • Scientists could not study this because they lacked the tools to see things this small. ...
... • Early scientists theorized that eventually you would not be able to cut it in half any more. o Only one particle would be left. o They named these particles ‘Atoms’ • Atoms means ‘cannot be divided’ • Scientists could not study this because they lacked the tools to see things this small. ...
Name - Madison County Schools
... Li – because it’s one valence electron is in energy level 2 which is close to the nucleus resulting in a much stronger magnetic pull on it than on the valence electrons of other members of the group which as in higher energy level. J. Define Electronegativity? Which element has the highest electrone ...
... Li – because it’s one valence electron is in energy level 2 which is close to the nucleus resulting in a much stronger magnetic pull on it than on the valence electrons of other members of the group which as in higher energy level. J. Define Electronegativity? Which element has the highest electrone ...
Drawing Atomic Structure
... Protons and Neutrons can both be broken down even farther into _______________. More info on pg. 508 ...
... Protons and Neutrons can both be broken down even farther into _______________. More info on pg. 508 ...
Name
... Atoms of different elements can _________________mix together or ___________________ combine. Chemical reactions occur when atoms are__________________, _________________, or _________________________. ...
... Atoms of different elements can _________________mix together or ___________________ combine. Chemical reactions occur when atoms are__________________, _________________, or _________________________. ...