Chemical Reactions Chemistry - is the study of matter, its properties
... Many chemicals can be hazardous to human health or the environment if they are not handled safely. There are a variety of symbols used to identify hazardous chemicals. Many household products are labeled with Hazardous Household Product Symbols (HHPS). Dangerous materials in the workplace are labele ...
... Many chemicals can be hazardous to human health or the environment if they are not handled safely. There are a variety of symbols used to identify hazardous chemicals. Many household products are labeled with Hazardous Household Product Symbols (HHPS). Dangerous materials in the workplace are labele ...
Theories and Structure of the Atom
... Most alpha particles passed straight through the foil A few alpha particles bounced back Rutherford’s Contributions to the atomic model Since most of the alpha particles passed through the foil: 1. Most of the atom is empty space Since some of the alpha particles bounced back: 2. Part of the ato ...
... Most alpha particles passed straight through the foil A few alpha particles bounced back Rutherford’s Contributions to the atomic model Since most of the alpha particles passed through the foil: 1. Most of the atom is empty space Since some of the alpha particles bounced back: 2. Part of the ato ...
the atom
... chemically into simpler substances. Hydrogen and oxygen are examples of elements. A compound when composed of two or more types of elements combined in a definite ratio, and can be decomposed by a chemical change into two or more other pure substances. Water is a compound composed of two parts hydro ...
... chemically into simpler substances. Hydrogen and oxygen are examples of elements. A compound when composed of two or more types of elements combined in a definite ratio, and can be decomposed by a chemical change into two or more other pure substances. Water is a compound composed of two parts hydro ...
Ch t 6 Chapter 6 Electronic Structure and the Periodic Table Outline
... • Recall that an orbital is a physical representation of the region in space where there is a 90% probability of finding an electron • We will consider the shapes of two types of orbitals in this chapter • s orbitals are spherical, differing only in size (they become larger as n increases) • p orbit ...
... • Recall that an orbital is a physical representation of the region in space where there is a 90% probability of finding an electron • We will consider the shapes of two types of orbitals in this chapter • s orbitals are spherical, differing only in size (they become larger as n increases) • p orbit ...
chapter6 ppt - Tolland High School
... Elements in Group 1 and 2 fill an s sublevel Elements in Groups 13-18 fill a p sublevel Elements of the transition metals fill a d sublevel The two sets of 14 elements each at the bottom of the periodic table fill f sublevels with a principal quantum number two less than the period number • First ro ...
... Elements in Group 1 and 2 fill an s sublevel Elements in Groups 13-18 fill a p sublevel Elements of the transition metals fill a d sublevel The two sets of 14 elements each at the bottom of the periodic table fill f sublevels with a principal quantum number two less than the period number • First ro ...
Chemistry IGCSE Revision PDF File
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
Standard 1:Atomic Structure + Elements, Compounds, Mixtures
... A substance is an element if all the atoms are the same color and size and they behave in the same way. ...
... A substance is an element if all the atoms are the same color and size and they behave in the same way. ...
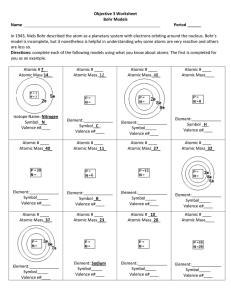
Objective 3 Worksheet Bohr Models Name Period In 1943, Niels
... Objective 3 Worksheet Bohr Models Name ...
... Objective 3 Worksheet Bohr Models Name ...
Unit 2 Spiraling
... Show work and box answers. All answers should have units and be to the correct number of significant figures: 9. How many grams are there in 1.50 moles of Na (sodium metal)? How many atoms in the same amount? 10. How many grams are there in 0.00150 moles of H2O? How many molecules in the same amount ...
... Show work and box answers. All answers should have units and be to the correct number of significant figures: 9. How many grams are there in 1.50 moles of Na (sodium metal)? How many atoms in the same amount? 10. How many grams are there in 0.00150 moles of H2O? How many molecules in the same amount ...
Chapter 9 - profpaz.com
... 2. Argon (Ar) has 18 protons, 18 electrons and 22 neutrons. Write a formula designation for an argon atom. Atomic number = Mass number = protons + neutrons = ...
... 2. Argon (Ar) has 18 protons, 18 electrons and 22 neutrons. Write a formula designation for an argon atom. Atomic number = Mass number = protons + neutrons = ...
Chemistry: Spring Semester Lecture Notes - Teach-n-Learn-Chem
... 2. Atoms of the same element are exactly alike; in particular, they have the same mass. 3. Compounds are formed by the joining of atoms of two or more elements in fixed, whole number ratios, e.g., ...
... 2. Atoms of the same element are exactly alike; in particular, they have the same mass. 3. Compounds are formed by the joining of atoms of two or more elements in fixed, whole number ratios, e.g., ...
Using your periodic table ppt (9/26-10/11) File
... isotopes. They are Ga-69 with a 60.108% abundance and a mass of 68.926 amu and Ga-71 with a 39.892% abundance and an atomic mass of 70.925. Calculate the atomic mass of gallium. 7. The atomic mass of bromine given in the periodic table is 79.904 amu, which is very close to 80 amu. Use a reference bo ...
... isotopes. They are Ga-69 with a 60.108% abundance and a mass of 68.926 amu and Ga-71 with a 39.892% abundance and an atomic mass of 70.925. Calculate the atomic mass of gallium. 7. The atomic mass of bromine given in the periodic table is 79.904 amu, which is very close to 80 amu. Use a reference bo ...
Atoms, Ions, and the Periodic Table
... The He nucleus was deflected or reflected and couldn’t move the nucleus 4. Do you think that, in Rutherford's experiment, the electrons in the gold atoms would deflect the alpha particles significantly? Why or why not? No, because the He nucleus has a much greater mass than an electron. 5. Rutherfor ...
... The He nucleus was deflected or reflected and couldn’t move the nucleus 4. Do you think that, in Rutherford's experiment, the electrons in the gold atoms would deflect the alpha particles significantly? Why or why not? No, because the He nucleus has a much greater mass than an electron. 5. Rutherfor ...
How many significant figures are there in each of these
... - Dalton's theory sets LIMITS on what can be done with chemistry. For example: Chemistry can't convert lead (an element) into gold (another element). Sorry, alchemists! You can't have a compound form in a chemical reaction that contains an element that was not in your starting materials. You can onl ...
... - Dalton's theory sets LIMITS on what can be done with chemistry. For example: Chemistry can't convert lead (an element) into gold (another element). Sorry, alchemists! You can't have a compound form in a chemical reaction that contains an element that was not in your starting materials. You can onl ...
Chapter 2 Atoms, Ions, and Molecules
... have identical physical and chemical properties 3. atoms of different elements have different masses, physical properties, and chemical properties 4. atoms of different elements combine in simple whole numbers to form compounds 5. atoms of an element cannot be converted into atoms of other elements; ...
... have identical physical and chemical properties 3. atoms of different elements have different masses, physical properties, and chemical properties 4. atoms of different elements combine in simple whole numbers to form compounds 5. atoms of an element cannot be converted into atoms of other elements; ...
elements and isotopes - vocabulary
... The average mass of all atoms of a particular element found in nature. It is also called relative atomic mass. It is expressed in atomic mass units (amu). On the atomic mass scale, the mass of one atom of carbon-12 is set up as a standard and is exactly 12 amu. naturally occurring isotope An isotope ...
... The average mass of all atoms of a particular element found in nature. It is also called relative atomic mass. It is expressed in atomic mass units (amu). On the atomic mass scale, the mass of one atom of carbon-12 is set up as a standard and is exactly 12 amu. naturally occurring isotope An isotope ...
Why are atoms of lead different to those of gold and why can we not
... His results showed that most of the gold foil is space as the radioactive particles went straight through the foil. This left little cirles where the gold atoms were. It was not until 1932 that we finally established the structure of the atom. Lets look at an atoms of helium. ...
... His results showed that most of the gold foil is space as the radioactive particles went straight through the foil. This left little cirles where the gold atoms were. It was not until 1932 that we finally established the structure of the atom. Lets look at an atoms of helium. ...
Atoms, Ions, and the Periodic Table
... The He nucleus was deflected or reflected and couldn’t move the nucleus 4. Do you think that, in Rutherford's experiment, the electrons in the gold atoms would deflect the alpha particles significantly? Why or why not? No, because the He nucleus has a much greater mass than an electron. 5. Rutherfor ...
... The He nucleus was deflected or reflected and couldn’t move the nucleus 4. Do you think that, in Rutherford's experiment, the electrons in the gold atoms would deflect the alpha particles significantly? Why or why not? No, because the He nucleus has a much greater mass than an electron. 5. Rutherfor ...
Unit 3 Powerpoint
... All are solids at room temp (except Mercury, which is a liquid) Metals tend to have low ionization energies, and typically lose electrons (i.e. are oxidized) when they undergo chemical reactions Alkali metals are always 1+ (lose the electron in s subshell) Alkaline earth metals are always 2+ (lose b ...
... All are solids at room temp (except Mercury, which is a liquid) Metals tend to have low ionization energies, and typically lose electrons (i.e. are oxidized) when they undergo chemical reactions Alkali metals are always 1+ (lose the electron in s subshell) Alkaline earth metals are always 2+ (lose b ...
Student Expectation
... Key Concept 1: During a chemical reaction, the atoms of substances rearrange themselves into a new configuration forming new substances. The reactants (or the energy and atoms or molecules of the original substance) combine to produce products (or the energy, atoms, and molecules of the new substanc ...
... Key Concept 1: During a chemical reaction, the atoms of substances rearrange themselves into a new configuration forming new substances. The reactants (or the energy and atoms or molecules of the original substance) combine to produce products (or the energy, atoms, and molecules of the new substanc ...
Atomic Theory - Mikus
... could not explain why this was the case. J.J. Thomson picked up on Dalton’s discoveries and added that each atom has electrons. He knew about this property, but he described that it was just a random mixture of positively and negatively charged particles. Rutherford picked up on Thomson’s experiment ...
... could not explain why this was the case. J.J. Thomson picked up on Dalton’s discoveries and added that each atom has electrons. He knew about this property, but he described that it was just a random mixture of positively and negatively charged particles. Rutherford picked up on Thomson’s experiment ...
Understanding the Atom
... Neutral particle in the nucleus is the neutron. Negatively charged particles that move in the space outside an atom’s nucleus is the electrons. ...
... Neutral particle in the nucleus is the neutron. Negatively charged particles that move in the space outside an atom’s nucleus is the electrons. ...
Atomic Structure
... which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
... which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...