Atomic Structure Problem Set PROBLEM SET #3: ATOMIC
... all isotopes of hydrogen? 1. proton 2. neutron 3. electron 4. positron ...
... all isotopes of hydrogen? 1. proton 2. neutron 3. electron 4. positron ...
No Slide Title
... • With this information, scientists were able to determine the mass of an electron. ...
... • With this information, scientists were able to determine the mass of an electron. ...
atom
... naturally occurring element on Earth is nearly always the same, no matter where the element is found. The percentage at which each of an element’s isotopes occurs in nature is taken into account when calculating the element’s average atomic mass ...
... naturally occurring element on Earth is nearly always the same, no matter where the element is found. The percentage at which each of an element’s isotopes occurs in nature is taken into account when calculating the element’s average atomic mass ...
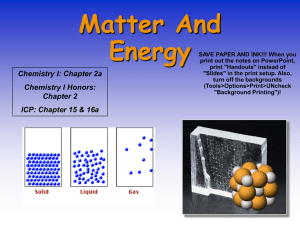
Matter and Energy
... • Extensive properties are dependent upon the amount of substance present. Ex- mass, length • Intensive property is independent of the amount of substance present. Ex- density, temperature ...
... • Extensive properties are dependent upon the amount of substance present. Ex- mass, length • Intensive property is independent of the amount of substance present. Ex- density, temperature ...
How many protons, electrons and neutrons are in an atom of krypton
... The interesting thing here is that adding or removing neutrons from an atom does not create a different element. Rather, it creates a heavier or lighter version of that element. These different versions are called isotopes and most elements are actually a mixture of different isotopes. If you could ...
... The interesting thing here is that adding or removing neutrons from an atom does not create a different element. Rather, it creates a heavier or lighter version of that element. These different versions are called isotopes and most elements are actually a mixture of different isotopes. If you could ...
Devil physics The baddest class on campus IB Physics
... force – the strong nuclear force The strong nuclear force is an attractive force and much stronger than the electrical force, if the separation between nucleons is kept very small (on the order of 10-15 m or less) For larger separations, the strong nuclear force becomes so small as to be negligi ...
... force – the strong nuclear force The strong nuclear force is an attractive force and much stronger than the electrical force, if the separation between nucleons is kept very small (on the order of 10-15 m or less) For larger separations, the strong nuclear force becomes so small as to be negligi ...
File
... amu). Based upon the average atomic mass of carbon (12.011 amu), which isotope of carbon do you think is the most abundant in nature? Explain your answer. An element has two naturally occurring isotopes. The mass of the first isotope is 64.9278 amu and the mass of the second isotope is 62.9296 amu. ...
... amu). Based upon the average atomic mass of carbon (12.011 amu), which isotope of carbon do you think is the most abundant in nature? Explain your answer. An element has two naturally occurring isotopes. The mass of the first isotope is 64.9278 amu and the mass of the second isotope is 62.9296 amu. ...
Atomic Basics
... Ground vs. Excited state for e-, then, Bright Line Emission Spectra Although Neils Bohr put the electrons into orbits, which was wrong, he did put them into energy levels. These levels are exact, and the electrons tend to stay in the lowest energy levels possible. That means that the orbitals (his o ...
... Ground vs. Excited state for e-, then, Bright Line Emission Spectra Although Neils Bohr put the electrons into orbits, which was wrong, he did put them into energy levels. These levels are exact, and the electrons tend to stay in the lowest energy levels possible. That means that the orbitals (his o ...
Atoms, Elements, and Ions
... Does not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have different atoms YES! 4. atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merel ...
... Does not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have different atoms YES! 4. atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merel ...
Using mass to calculate molecular formula
... Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number of atoms in the molecule. Percentages of ...
... Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number of atoms in the molecule. Percentages of ...
unit 2 - chemistry
... b. 4 basic – H, C, O, N, -96% of human mass Ca, P -> 99% (3%) K, S, Cl, Mg, I, FE, (16 other)1% -> ...
... b. 4 basic – H, C, O, N, -96% of human mass Ca, P -> 99% (3%) K, S, Cl, Mg, I, FE, (16 other)1% -> ...
Chemistry: Matter and Change
... • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
... • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
Test 3 Review
... Wave Mechanical Model. The Bohr model successfully explained the bright line spectra for hydrogen, but could not explain the spectra of atoms with more electrons. The wave mechanical model solved the problem. The wave mechanical model describes the location of electrons a their most probable locatio ...
... Wave Mechanical Model. The Bohr model successfully explained the bright line spectra for hydrogen, but could not explain the spectra of atoms with more electrons. The wave mechanical model solved the problem. The wave mechanical model describes the location of electrons a their most probable locatio ...
Bohr Diagram - hrsbstaff.ednet.ns.ca
... An ion of chlorine must also have _______ electrons because it is more stable. This results in chlorine having a net charge of 1-. The name is __________ chloride ...
... An ion of chlorine must also have _______ electrons because it is more stable. This results in chlorine having a net charge of 1-. The name is __________ chloride ...
atoms - Westerly High School
... times greater than the diameter of its nucleus * If the nucleus were the size of the period at the end of this sentence, the outer edges of the atom would ...
... times greater than the diameter of its nucleus * If the nucleus were the size of the period at the end of this sentence, the outer edges of the atom would ...
Masses of Atoms
... 80% of Boron in nature have 5 protons, 6 neutrons ~ 11 amu 20% of Boron in nature have 5 protons, 5 neutrons ~ 10 amu (.8 • 11 amu) + (.2 • 10 amu) = 8.8 amu + 2 amu = 10.8 amu There are a few extra isotopes out there that we did not include. ...
... 80% of Boron in nature have 5 protons, 6 neutrons ~ 11 amu 20% of Boron in nature have 5 protons, 5 neutrons ~ 10 amu (.8 • 11 amu) + (.2 • 10 amu) = 8.8 amu + 2 amu = 10.8 amu There are a few extra isotopes out there that we did not include. ...
Gizmo Lab Bohr Models 2014
... Student Exploration: Element Builder Investigating Atoms and Drawing Bohr Models Vocabulary: atom, atomic number, electron, element, energy level, mass number, neutron, nucleus, Periodic Table, proton, valence electrons Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. What are some of ...
... Student Exploration: Element Builder Investigating Atoms and Drawing Bohr Models Vocabulary: atom, atomic number, electron, element, energy level, mass number, neutron, nucleus, Periodic Table, proton, valence electrons Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. What are some of ...
The Atom Review Packet
... 7. The total number of electrons in a neutral atom of every element is always equal to the atom’s (1) mass number (2) number of neutrons (3) number of protons (4) number of nucleons 8. The atomic number of an atom is equal to the number of (1) neutrons in the atom (2) protons in the atom (3) neutron ...
... 7. The total number of electrons in a neutral atom of every element is always equal to the atom’s (1) mass number (2) number of neutrons (3) number of protons (4) number of nucleons 8. The atomic number of an atom is equal to the number of (1) neutrons in the atom (2) protons in the atom (3) neutron ...
CHM 103 Lecture 11 S07
... • collisions between molecules have sufficient energy to break the bonds in the reactants. • bonds between atoms of the reactants (N2 and O2) are broken and new bonds (NO) can form. Cu(s) Orange metal Ag1+(aq) + 2eColorless ...
... • collisions between molecules have sufficient energy to break the bonds in the reactants. • bonds between atoms of the reactants (N2 and O2) are broken and new bonds (NO) can form. Cu(s) Orange metal Ag1+(aq) + 2eColorless ...
UC Irvine FOCUS! 5 E Lesson Plan Title: Marble Isotope Lab Grade
... Different atoms of the same element can have different numbers of neutrons. Because the protons and neutrons make up the mass of the atom, different atoms of the same element can have different masses! These are called isotopes. For example: Lithium (Li) has two isotopes. Both isotopes have 3 proton ...
... Different atoms of the same element can have different numbers of neutrons. Because the protons and neutrons make up the mass of the atom, different atoms of the same element can have different masses! These are called isotopes. For example: Lithium (Li) has two isotopes. Both isotopes have 3 proton ...
Intro to atoms and the periodic table
... Atoms - very, very small Can’t be seen w/ visible light Smaller than wavelengths of visible light ...
... Atoms - very, very small Can’t be seen w/ visible light Smaller than wavelengths of visible light ...