Answer - Test Bank wizard
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
types of reactions
... limiting reactant: reactant which is depleated first (not the one with the lesser amount) • concentration of reactants is important because if we run out of one of the reactants it can limit and stop the whole reaction ex: How many smores can be made with the following? ...
... limiting reactant: reactant which is depleated first (not the one with the lesser amount) • concentration of reactants is important because if we run out of one of the reactants it can limit and stop the whole reaction ex: How many smores can be made with the following? ...
10/9 atomic structure powerpoint 2
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
Chapter 4
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
Answer - TEST BANK 360
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
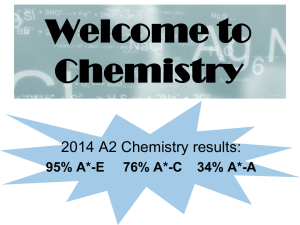
Welcome to Chemistry
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
Atomic structure Atomic masses
... Relative atomic mass, Ar: the weighted mean mass of an atom of an element compared with 1/12 of the the mass of an atom of carbon-12 The term ‘weighted mean mass’ is used to account for the contribution made by each isotope to the overall mass of an element. The contribution made by an isotope to th ...
... Relative atomic mass, Ar: the weighted mean mass of an atom of an element compared with 1/12 of the the mass of an atom of carbon-12 The term ‘weighted mean mass’ is used to account for the contribution made by each isotope to the overall mass of an element. The contribution made by an isotope to th ...
Chapter 20: Electrochemistry
... -Assign each element an Oxidation Number (State). If any element changes it’s oxidation state during a reaction, it is a redox reaction Zero Oxidation States indicate element is neutral [+] Oxidation States indicate element is “electron poor” [-] Oxidation States indicate element is “electron rich” ...
... -Assign each element an Oxidation Number (State). If any element changes it’s oxidation state during a reaction, it is a redox reaction Zero Oxidation States indicate element is neutral [+] Oxidation States indicate element is “electron poor” [-] Oxidation States indicate element is “electron rich” ...
Section 1-2 Matter and Its Properties
... A chemical change is also known as a chemical reaction. The substances that react in a chemical change are called the reactants. The substances that are formed by the chemical change are called the products. In burning charcoal (carbon), the carbon mixes with oxygen to form carbon dioxide. ...
... A chemical change is also known as a chemical reaction. The substances that react in a chemical change are called the reactants. The substances that are formed by the chemical change are called the products. In burning charcoal (carbon), the carbon mixes with oxygen to form carbon dioxide. ...
What is an atom? What are atoms made up from? Do we really touch
... reflected backwards as well. Rutherford concluded from these observations that the atom is mostly consisted of empty space since the positively charged particles travelled through the gold foil without a deflection, regarding the small portion of alpha particles that were deflected and reflected, h ...
... reflected backwards as well. Rutherford concluded from these observations that the atom is mostly consisted of empty space since the positively charged particles travelled through the gold foil without a deflection, regarding the small portion of alpha particles that were deflected and reflected, h ...
New substances are formed by chemical reactions. When elements
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
File
... Dalton's Atomic Theory 3. Atoms of two or more different elements combine to form compounds. A particular compound is always made up of the same kinds of atoms and the same number of each kind of atom. 4. A chemical reaction involves the rearrangement, separation, or combination of atoms. Atoms are ...
... Dalton's Atomic Theory 3. Atoms of two or more different elements combine to form compounds. A particular compound is always made up of the same kinds of atoms and the same number of each kind of atom. 4. A chemical reaction involves the rearrangement, separation, or combination of atoms. Atoms are ...
Atomic Physics Sections 9.1-9.7
... elements that compose it. For example, when water is broken down by electrolysis into oxygen and hydrogen, the mass ratio is always 8 to 1. ...
... elements that compose it. For example, when water is broken down by electrolysis into oxygen and hydrogen, the mass ratio is always 8 to 1. ...
drawing bohr models
... Bohr’s Atomic Theory Ex.3) Draw a Bohr model of Lithium-3. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw circles around the nucleus to represent the electron shells. Step-4 Place the electrons in the she ...
... Bohr’s Atomic Theory Ex.3) Draw a Bohr model of Lithium-3. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw circles around the nucleus to represent the electron shells. Step-4 Place the electrons in the she ...
Metals
... exist as an array of ions or atoms bound to each other but with no recognisable molecules. The formula NaCl instead tells us that throughout a sample of NaCl sodium and chlorine atoms are present in the ratio 1:1. Because ionic compounds do not contain molecules the sum of the relative atomic masses ...
... exist as an array of ions or atoms bound to each other but with no recognisable molecules. The formula NaCl instead tells us that throughout a sample of NaCl sodium and chlorine atoms are present in the ratio 1:1. Because ionic compounds do not contain molecules the sum of the relative atomic masses ...
Combustion Of Alcohols Essay, Research Paper Comparing Energy
... I predicted. The reason that my readings for energy release increased with increased molecular weight is because as the number of bonds broken then made increases (with increased molecular weight of the alcohol) then there is proportionately more exothermic energy released. The higher molecular weig ...
... I predicted. The reason that my readings for energy release increased with increased molecular weight is because as the number of bonds broken then made increases (with increased molecular weight of the alcohol) then there is proportionately more exothermic energy released. The higher molecular weig ...
Atoms - Mrs. Lindenlaub
... Since Democritus had the idea that these small particles were indivisible, they became known as atomos… ...
... Since Democritus had the idea that these small particles were indivisible, they became known as atomos… ...
Descriptive Chemistry of Elements d-Block
... Note that the 4s-electrons were removed before the removal of the 3d-electrons. The reason for this is that in a metal cation the energy of 3d-orbitals is lower than the energy of 4s-orbitals (c.f. when filling electrons, the energy of the 4s orbital is lower than 3d-orbitals). Thus the valence elec ...
... Note that the 4s-electrons were removed before the removal of the 3d-electrons. The reason for this is that in a metal cation the energy of 3d-orbitals is lower than the energy of 4s-orbitals (c.f. when filling electrons, the energy of the 4s orbital is lower than 3d-orbitals). Thus the valence elec ...
69. (M) Each atom of F contains 9 protons (1.0073 u each), 10
... This compares with a mass of 18.9984 u given in the periodic table. The difference, 0.160 u per atom, is called the mass defect and represents the energy that holds the nucleus together, the nuclear binding energy. This binding energy is released when 9 protons and 9 neutrons fuse to give a fluorine ...
... This compares with a mass of 18.9984 u given in the periodic table. The difference, 0.160 u per atom, is called the mass defect and represents the energy that holds the nucleus together, the nuclear binding energy. This binding energy is released when 9 protons and 9 neutrons fuse to give a fluorine ...
Fall.2008.Week9.Lesson.2 - reich
... • 1-Berylium chloride and aluminum react together. What is the reaction type? Balance the chemical reaction. • 2-Magnesium chloride and sodium phosphate undergo a double displacement reaction. Go through all the steps to show the net ionic equation. • 3- When you cook with a propane grill you burn p ...
... • 1-Berylium chloride and aluminum react together. What is the reaction type? Balance the chemical reaction. • 2-Magnesium chloride and sodium phosphate undergo a double displacement reaction. Go through all the steps to show the net ionic equation. • 3- When you cook with a propane grill you burn p ...
Aqueous Reactions
... equations, which show the compounds as individual ions. Until now, you have been writing chemical equations in this form: Pb(NO 3 ) 2 (aq) + 2KI(aq) Æ PbI 2 (s) + 2KNO 3 (aq) Equations written this way are known as molecular equations. They have a variation known as a complete ionic equation, in whi ...
... equations, which show the compounds as individual ions. Until now, you have been writing chemical equations in this form: Pb(NO 3 ) 2 (aq) + 2KI(aq) Æ PbI 2 (s) + 2KNO 3 (aq) Equations written this way are known as molecular equations. They have a variation known as a complete ionic equation, in whi ...
Answer - Test Bank 1
... charged parts of an ionic compound. T 63. (T/F) Atoms of the same element can possess a different number of protons. F 64. (T/F) Using isotopic notation, the number of neutrons in an atom is contained within the mass number. T 65. Electrostatic attractive forces, used to form ionic compounds, exist ...
... charged parts of an ionic compound. T 63. (T/F) Atoms of the same element can possess a different number of protons. F 64. (T/F) Using isotopic notation, the number of neutrons in an atom is contained within the mass number. T 65. Electrostatic attractive forces, used to form ionic compounds, exist ...
Precipitation Reactions
... The rules you just learned assume that the redox reaction is taking place under acidic conditions. (You are, after all, either producing or consuming H+ ions.) There are slightly different rules for basic conditions: 1. Balance the reaction (using your method of choice) as if it were under acidic co ...
... The rules you just learned assume that the redox reaction is taking place under acidic conditions. (You are, after all, either producing or consuming H+ ions.) There are slightly different rules for basic conditions: 1. Balance the reaction (using your method of choice) as if it were under acidic co ...