Introduction to particle physics
... Gellmann (1961): arranged particles into patterns using group theory The eightfold way Predicts new particle (Ω-); mass and charge ...
... Gellmann (1961): arranged particles into patterns using group theory The eightfold way Predicts new particle (Ω-); mass and charge ...
KUT 203/2 - Chemistry Practical III (Inorganic Chemistry)
... • Substantiate the optical behaviour of these isomers. • Understand the concept on lingkage isomerism in coordination compounds and the technique used in the synthesis and isolation of these complex ions i.e. [(NH3)5CoNO2]Cl2 and [(NH3)5CoONO]Cl2. • Substantiate the functional group present in the i ...
... • Substantiate the optical behaviour of these isomers. • Understand the concept on lingkage isomerism in coordination compounds and the technique used in the synthesis and isolation of these complex ions i.e. [(NH3)5CoNO2]Cl2 and [(NH3)5CoONO]Cl2. • Substantiate the functional group present in the i ...
H2, N2, O2, F2, Cl2, Br2, I2
... 3. • Only change the coefficient ( the number in front of the formula ) when balancing. This tells us how many of each molecule or atom we have in the balanced equation. If there is no number in front, a " 1 " is there but we usually leave out the 1's. • Do not change subscripts to balance. They are ...
... 3. • Only change the coefficient ( the number in front of the formula ) when balancing. This tells us how many of each molecule or atom we have in the balanced equation. If there is no number in front, a " 1 " is there but we usually leave out the 1's. • Do not change subscripts to balance. They are ...
Chapter 3 Notes
... that contains as many atoms, molecules, ions, or other elementary units as the number of atoms in 12.01 g C. The number is 6.02 × 1023, or Avogadro's number. ...
... that contains as many atoms, molecules, ions, or other elementary units as the number of atoms in 12.01 g C. The number is 6.02 × 1023, or Avogadro's number. ...
09/11/03 lecture
... • Some elements can have a number of different possible atomic structures which differ only in the number of neutrons in the nucleus--isotopes. • For example, there are three different atomic structures for C : 12C, 13C, and 14 C. • When dealing with bulk quantities of carbon, more useful to think o ...
... • Some elements can have a number of different possible atomic structures which differ only in the number of neutrons in the nucleus--isotopes. • For example, there are three different atomic structures for C : 12C, 13C, and 14 C. • When dealing with bulk quantities of carbon, more useful to think o ...
CHEM-1411 Final Practice Exam
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
1411FINALSAMPLE+KEY - Houston Community College
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
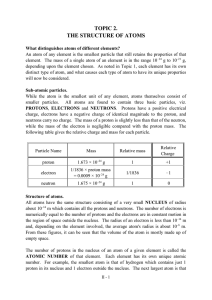
TOPIC 2. THE STRUCTURE OF ATOMS
... As atoms are electrically neutral and the charge on the electron is equal but opposite to that on the proton, there must be identical numbers of electrons and protons in any atom. The electrons are envisaged as being in rapid motion distributed around the nucleus, but never actually being within the ...
... As atoms are electrically neutral and the charge on the electron is equal but opposite to that on the proton, there must be identical numbers of electrons and protons in any atom. The electrons are envisaged as being in rapid motion distributed around the nucleus, but never actually being within the ...
File
... 53. In the diagram above, nitrogen atoms are represented as filled circles and oxygen atoms as open circles. How much NO2 can be prepared from the mixture shown? A) 4 molecules B) 5 molecules C) 6 molecules D) 8 molecules. 54. In which species is the electron geometry around the central atom tetrah ...
... 53. In the diagram above, nitrogen atoms are represented as filled circles and oxygen atoms as open circles. How much NO2 can be prepared from the mixture shown? A) 4 molecules B) 5 molecules C) 6 molecules D) 8 molecules. 54. In which species is the electron geometry around the central atom tetrah ...
Unit 2 Complete 2016 2017
... 6)______________________ The one element that has an isotope that does not contain all of the subatomic particles.(Hint: lightest element) 7)______________________ Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other elem ...
... 6)______________________ The one element that has an isotope that does not contain all of the subatomic particles.(Hint: lightest element) 7)______________________ Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other elem ...
TOPIC 2. THE STRUCTURE OF ATOMS
... than a noble gas atom, and they all behave as does sodium in that relatively little energy is needed to form their +1 cations Li+, Na+, K+, Rb+ and Cs+. Each of these cations has the same electron arrangement as the atom of the noble gas whose atomic number is 1 less than that of the first group ele ...
... than a noble gas atom, and they all behave as does sodium in that relatively little energy is needed to form their +1 cations Li+, Na+, K+, Rb+ and Cs+. Each of these cations has the same electron arrangement as the atom of the noble gas whose atomic number is 1 less than that of the first group ele ...
Chapter 4 PowerPoint - Southeast Online
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
Chapter 7-8-9
... a. a cation and an anion c. the ions of two different metals b. valence electrons and d. the ions of two cations different nonmetals Which of the following is true about the melting temperature of potassium chloride? a. The melting temperature is relatively high. b. The melting temperature is variab ...
... a. a cation and an anion c. the ions of two different metals b. valence electrons and d. the ions of two cations different nonmetals Which of the following is true about the melting temperature of potassium chloride? a. The melting temperature is relatively high. b. The melting temperature is variab ...
Honors Chemistry Atomic Theory Reading
... A man named John Dalton, (to the left) discovered this limitation in the law of definite proportions in some of his experiments. Dalton was experimenting with several reactions in which the reactant elements formed different products, depending on the experimental conditions he used. One common reac ...
... A man named John Dalton, (to the left) discovered this limitation in the law of definite proportions in some of his experiments. Dalton was experimenting with several reactions in which the reactant elements formed different products, depending on the experimental conditions he used. One common reac ...
Stuff Matters Handout
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
local section exam
... This test is designed to be taken with an answer sheet on which the student records his or her responses. All answers are to be marked on that sheet, not written in the booklet. Each student should be provided with an answer sheet and scratch paper, both of which must be turned in with the test book ...
... This test is designed to be taken with an answer sheet on which the student records his or her responses. All answers are to be marked on that sheet, not written in the booklet. Each student should be provided with an answer sheet and scratch paper, both of which must be turned in with the test book ...
PPT: Chemical Reactions Review
... Temperature at which reaction is carried out, in this case 0 oC ...
... Temperature at which reaction is carried out, in this case 0 oC ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
AP Chemistry - Oak Park Unified School District
... The energies of the (4) sublevels are less than the energy of the next higher s sublevel, whereas the energies of the (5) sublevels are greater than the next higher s sublevel. This restricts the outermost occupied sublevels for any atom to s and p. Electrons that occupy the outermost sublevels are ...
... The energies of the (4) sublevels are less than the energy of the next higher s sublevel, whereas the energies of the (5) sublevels are greater than the next higher s sublevel. This restricts the outermost occupied sublevels for any atom to s and p. Electrons that occupy the outermost sublevels are ...
Chapter 4
... ■ Teacher- summarized results of his experiments and those of others. ■ Elements substances that can’t be broken down ■ In Dalton’s Atomic Theory ■ Combined idea of elements with that of atoms. ...
... ■ Teacher- summarized results of his experiments and those of others. ■ Elements substances that can’t be broken down ■ In Dalton’s Atomic Theory ■ Combined idea of elements with that of atoms. ...