Polarity of Molecules
... VB model successful in explaining bonding in diatomics in terms of atomic orbitals……. ...
... VB model successful in explaining bonding in diatomics in terms of atomic orbitals……. ...
Endothermic reactions
... inside a glow stick, shown in Figure 19, is an example of an exergonic reaction, which produces visible light. In other reactions, however, energy is released as heat. This is the case with some heat packs that are used to treat muscle aches and other medical conditions. Another release of energy is ...
... inside a glow stick, shown in Figure 19, is an example of an exergonic reaction, which produces visible light. In other reactions, however, energy is released as heat. This is the case with some heat packs that are used to treat muscle aches and other medical conditions. Another release of energy is ...
Chemical Reactions and The Mole
... By definition an AMU is 1/12th the mass of a C-12 atom. The unit of mass needs a reference point and a specific amount of matter to which all other matter can be referenced. This is a standard, as C-12 is very abundant, but this mass is very small, too small to work with. Generally, you will work wi ...
... By definition an AMU is 1/12th the mass of a C-12 atom. The unit of mass needs a reference point and a specific amount of matter to which all other matter can be referenced. This is a standard, as C-12 is very abundant, but this mass is very small, too small to work with. Generally, you will work wi ...
Section 3 Electron Configurations
... • Explain the mathematical relationship among the speed, wavelength, and frequency of electromagnetic radiation. • Discuss the dual wave-particle nature of light. • Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. ...
... • Explain the mathematical relationship among the speed, wavelength, and frequency of electromagnetic radiation. • Discuss the dual wave-particle nature of light. • Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. ...
From Last Time… Electron diffraction Making a particle out of waves
... • Atoms do emit radiation, but only at certain discrete frequencies. • Frequencies are unique for different atoms • Spectrum is an atomic ‘fingerprint’, used to identify atoms (e.g. in space). ...
... • Atoms do emit radiation, but only at certain discrete frequencies. • Frequencies are unique for different atoms • Spectrum is an atomic ‘fingerprint’, used to identify atoms (e.g. in space). ...
Atoms, Ions and Molecules
... properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. 3. Compounds are formed when atoms of two or more elements combine. In a given compound, the relative numbers of at ...
... properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. 3. Compounds are formed when atoms of two or more elements combine. In a given compound, the relative numbers of at ...
Second exam 2014 with answers
... Last Name: ____________________________________________ First Name: _____________________________________________ Note: There are 10 questions in this exam (check both sides of the sheet). Fill in your answer in the blank space provided immediately following each question. 1/2 point will be subtract ...
... Last Name: ____________________________________________ First Name: _____________________________________________ Note: There are 10 questions in this exam (check both sides of the sheet). Fill in your answer in the blank space provided immediately following each question. 1/2 point will be subtract ...
Chapter 7 Chemical Formulas
... write the symbols for the ions side by side, cations first: Al3+ O22. Cross over the charges by using the absolute value of each ion’s charge as the subscript for the other ion: Al23+ O3-2 3. Check the subscripts and simplify if necessary. Final answer = Al2 O3 ...
... write the symbols for the ions side by side, cations first: Al3+ O22. Cross over the charges by using the absolute value of each ion’s charge as the subscript for the other ion: Al23+ O3-2 3. Check the subscripts and simplify if necessary. Final answer = Al2 O3 ...
10 Chemistry
... 1. Atomic number (Z) – number of protons in the nucleus of an atom – neutral atom: number of protons = number of electrons ...
... 1. Atomic number (Z) – number of protons in the nucleus of an atom – neutral atom: number of protons = number of electrons ...
This question is about the elements in Period 3 of the Periodic Table
... A TOF mass spectrometer can be used to determine the relative molecular mass of molecular substances. Explain why it is necessary to ionise molecules when measuring their mass in a TOF mass spectrometer. ...
... A TOF mass spectrometer can be used to determine the relative molecular mass of molecular substances. Explain why it is necessary to ionise molecules when measuring their mass in a TOF mass spectrometer. ...
Bonding and Nomenclature
... The molecule must contain polar bonds This can be determined from differences in electronegativity. Symmetry can not cancel out the effects of the polar bonds. Must determine geometry first. ...
... The molecule must contain polar bonds This can be determined from differences in electronegativity. Symmetry can not cancel out the effects of the polar bonds. Must determine geometry first. ...
The Structure of Atoms
... Composition of the Atomic Nucleus • Except for the nucleus of the simplest type of hydrogen atom, all atomic nuclei are made of protons and neutrons. • A proton has a positive charge equal in magnitude to the negative charge of an electron. • Atoms are electrically neutral because they contain equal ...
... Composition of the Atomic Nucleus • Except for the nucleus of the simplest type of hydrogen atom, all atomic nuclei are made of protons and neutrons. • A proton has a positive charge equal in magnitude to the negative charge of an electron. • Atoms are electrically neutral because they contain equal ...
Chapter 3 - Whitwell High School
... To balance, we place counting numbers in front of the formulas until the equation is balanced. ...
... To balance, we place counting numbers in front of the formulas until the equation is balanced. ...
PowerPoint
... Oxidation-Reduction Reactions • “Redox Reactions” – Involve the transfer of one or more electrons from one substance to another – Examples • Formation of compounds from its elements and vice versa • Combustion reactions • Reactions that produce electricity in batteries • Cellular Respiration (energ ...
... Oxidation-Reduction Reactions • “Redox Reactions” – Involve the transfer of one or more electrons from one substance to another – Examples • Formation of compounds from its elements and vice versa • Combustion reactions • Reactions that produce electricity in batteries • Cellular Respiration (energ ...
A Journey Through Time The Atom Introduction: If you were asked to
... that have led to our current knowledge of the structure of the atom. Using your research, you will construct a virtual timeline as a visual representation of the development of modern atomic theory. Using Microsoft Powerpoint, you can create a slideshow in increasing chronological order representing ...
... that have led to our current knowledge of the structure of the atom. Using your research, you will construct a virtual timeline as a visual representation of the development of modern atomic theory. Using Microsoft Powerpoint, you can create a slideshow in increasing chronological order representing ...
chemistry - Textbooks Online
... the branch of science we call chemistry we have gained an understanding of the matter which makes up our world and of the interactions between particles on which it depends. The ancient Greek philosophers had their own ideas of the nature of matter, proposing atoms as the smallest indivisible partic ...
... the branch of science we call chemistry we have gained an understanding of the matter which makes up our world and of the interactions between particles on which it depends. The ancient Greek philosophers had their own ideas of the nature of matter, proposing atoms as the smallest indivisible partic ...
Medical Imaging
... Eo IK, L, M,... (resonance) Photoelectric absorption cross section decreases strongly with photon energy ( Ep-3) as photon energy increases relative to IK, L, M,... Photoelectric absorption cross section increases strongly with Z (~ Z3) because I Z Photoelectric absorption in K shell usually do ...
... Eo IK, L, M,... (resonance) Photoelectric absorption cross section decreases strongly with photon energy ( Ep-3) as photon energy increases relative to IK, L, M,... Photoelectric absorption cross section increases strongly with Z (~ Z3) because I Z Photoelectric absorption in K shell usually do ...
Atomic Structure
... individual particles called atoms. • His atomic theory of matter contains four hypotheses: 1. All matter is composed of tiny particles called atoms. 2. All atoms of an element are identical in mass and fundamental chemical properties. 3. A chemical compound is a substance that always contains the sa ...
... individual particles called atoms. • His atomic theory of matter contains four hypotheses: 1. All matter is composed of tiny particles called atoms. 2. All atoms of an element are identical in mass and fundamental chemical properties. 3. A chemical compound is a substance that always contains the sa ...
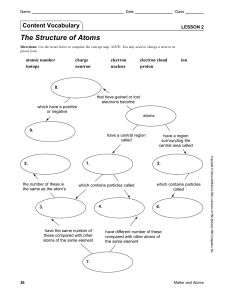
Lesson 2 | The Structure of Atoms
... Lesson 2: The Structure of Atoms A. The Parts of an Atom 1. Every kind of element is made up of its own kind of atoms. 2. Atoms are composed of several basic types of very small particles; the number of each of these particles gives the different kinds of atoms their unique identity. 3. The region ...
... Lesson 2: The Structure of Atoms A. The Parts of an Atom 1. Every kind of element is made up of its own kind of atoms. 2. Atoms are composed of several basic types of very small particles; the number of each of these particles gives the different kinds of atoms their unique identity. 3. The region ...